Protocol for the Invisalign Palatal Expander
After almost nine years of development, the Invisalign Palatal Expander* (IPE) can now be prescribed for maxillary expansion in children, adolescents, and adults (Fig. 1). This expansion system was approved by Health Canada (issued November 2021, amended October 2023) and the European Union (CE mark, November 2024), and in December 2023, it received 501(k) clearance from the U.S. Food and Drug Administration (FDA), which determined that it was substantially equivalent to other legally marketed devices for the same clinical indication.1-3
The IPE system consists of a series of staged, removable expanders designed to expand the maxilla and maxillary dentition in .25mm increments (Fig. 2). As with conventional expanders in growing patients, the increase in maxillary width is accomplished through skeletal and dental movements that separate the midpalatal suture.4-6 Each expander is directly printed from polyamide-12 (Nylon 12), eliminating the need to make models for fabrication. While light in weight, the expanders are rigid, generally lacking torsional flexibility—although the buccal flanks retain some flexibility to enable removal, assisted by built-in handles on the buccal sides of the molars.
With its transpalatal design, the IPE contacts the lateral palatal walls but not the top of the palate. The expanders are .75mm thick at the buccal surfaces and 1.5mm at the occlusal surfaces, while the thickness in the palatal region ranges from 2.5mm to 3mm. The trays are designed to cover the complete clinical crowns of the upper permanent molars and either the second deciduous molars or second premolars, as well as half of the first deciduous molars or first premolars. Therefore, simultaneous treatment with upper clear aligners or fixed appliances is not currently possible.
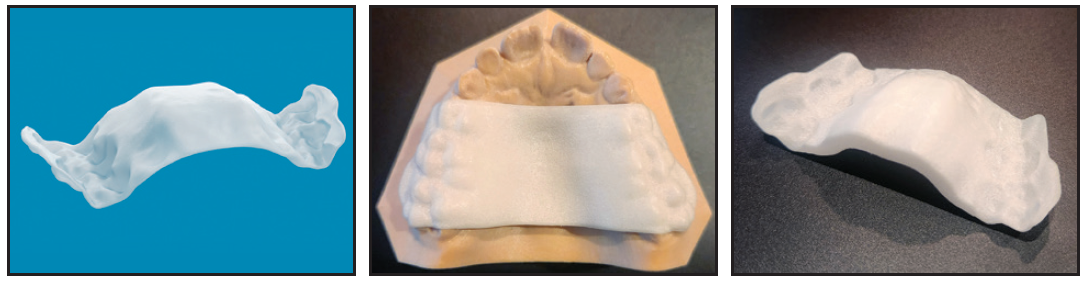
Fig. 1 Invisalign Palatal Expander* (IPE).
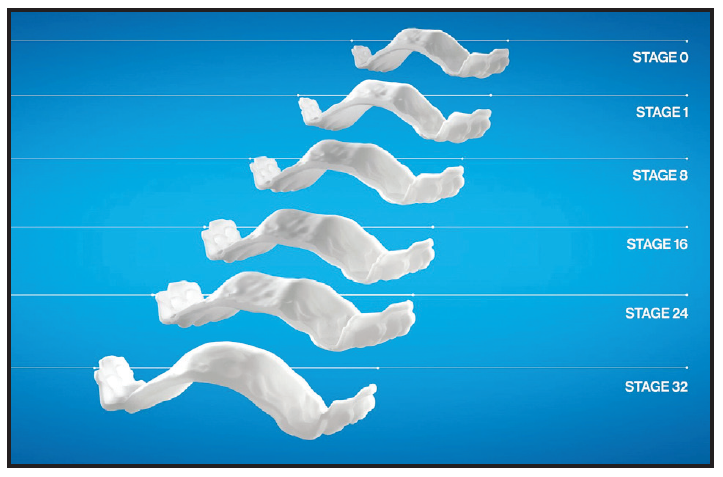
Fig. 2 Gradual increase in transverse dimension by stage.
Patient Selection and Prescription
The clinical indications for the IPE are the same as for traditional palatal expanders. To determine whether a patient meets the selection criteria for IPE treatment, a digital scan must capture the entire palate as far as a line connecting the distal surfaces of the upper first permanent molars or terminal molars. Manufacturing requirements specify that each quadrant must have at least three stable teeth, with minimum measurements of 4mm in vertical clinical crown height and 3mm of gingiva, captured in the distobuccal flange of the scan. Patients with short clinical crowns or stainless steel crowns on the first permanent molars or second deciduous molars do not meet the selection criteria.
When prescribing the IPE, the clinician decides how much expansion is required, based on the diagnosis and treatment plan, and then simply enters the desired amount of expansion into the online prescription form (Fig. 3). Currently, there is no visual treatment plan to approve. The practitioner may consider prescribing slightly more expansion than needed and using the patient’s response to determine whether to dispense the additional expanders.
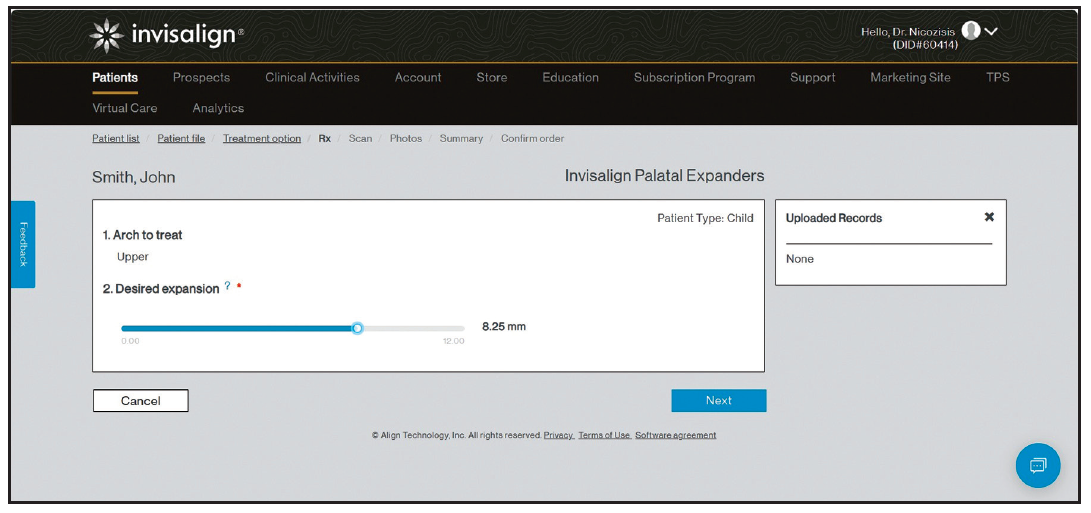
Fig. 3 Online prescription form includes slider for desired amount of expansion.
Installation and Use
Upon delivery of the expanders, attachments are bonded to improve mechanical retention. IPE attachments are conventional (as opposed to optimized), with no preactivated surfaces or SmartForce* features; they have a curved, semicircular shape without corners and are beveled on the gingival side. Following the protocol typically used for aligner attachments, the attachments are bonded to the upper first permanent molars and the second premolars or second deciduous molars, if present.
Patients are instructed to wear their IPEs full-time, even while eating, and to remove them only during brushing and flossing. Expanders should be changed daily for rapid expansion or less frequently for slower expansion, at the orthodontist’s discretion.
After the desired amount of expansion has been achieved, the patient is scanned for holding expanders, which are also worn full-time except when brushing and flossing. The doctor’s prescription for the length of the maintenance phase and the desired frequency of changes will determine the number of holding expanders that are delivered; for example, if the expansion needs to be maintained for six months with weekly changes, 24 holding expanders will be fabricated, but if the holding expanders are to be changed every two weeks, only 12 will be made.
Case 1
A 9-year-old male presented with the chief concerns of crowding and potentially blocked-out canines (Fig. 4). Palatal expansion and extraction of all deciduous canines were recommended as a first phase of treatment. Eventual alignment of the upper anterior teeth was planned to open space for the upper permanent canines, in the hope that they would erupt spontaneously. The lower arch was to be monitored for later use of the leeway space to resolve the lower crowding during comprehensive treatment.
A series of 34 IPEs was prescribed, to be changed daily. Upon completion of the expansion, the patient was scanned for holding expanders, which were worn for six months as the suture matured.
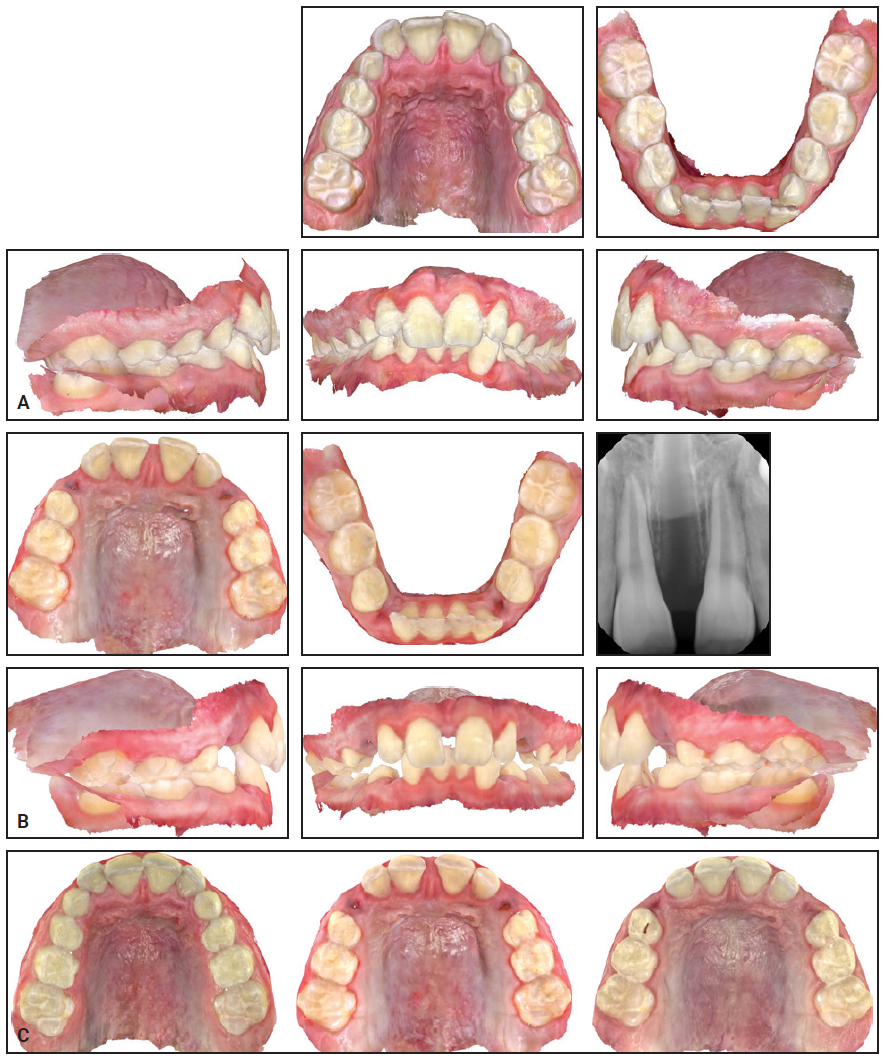
Fig. 4 Case 1. A. At beginning of treatment. B. After 33 of 34 stages of expansion with IPE. C. Progress of expansion: beginning of treatment (left), after expansion (center), and after four months of retention (right).
Case 2
An 8-year-old female presented with maxillary constriction and blocked-out upper lateral incisors (Fig. 5). The panoramic radiograph revealed insufficient space for the developing upper canines. Maxillary expansion was recommended as a first phase of treatment to facilitate alignment of the upper incisors and create more space for the permanent canines.
After the patient wore 32 of 34 prescribed IPEs, which were changed daily, a periapical radiograph demonstrated sutural expansion. Holding expanders were then worn for six months to maintain the results.
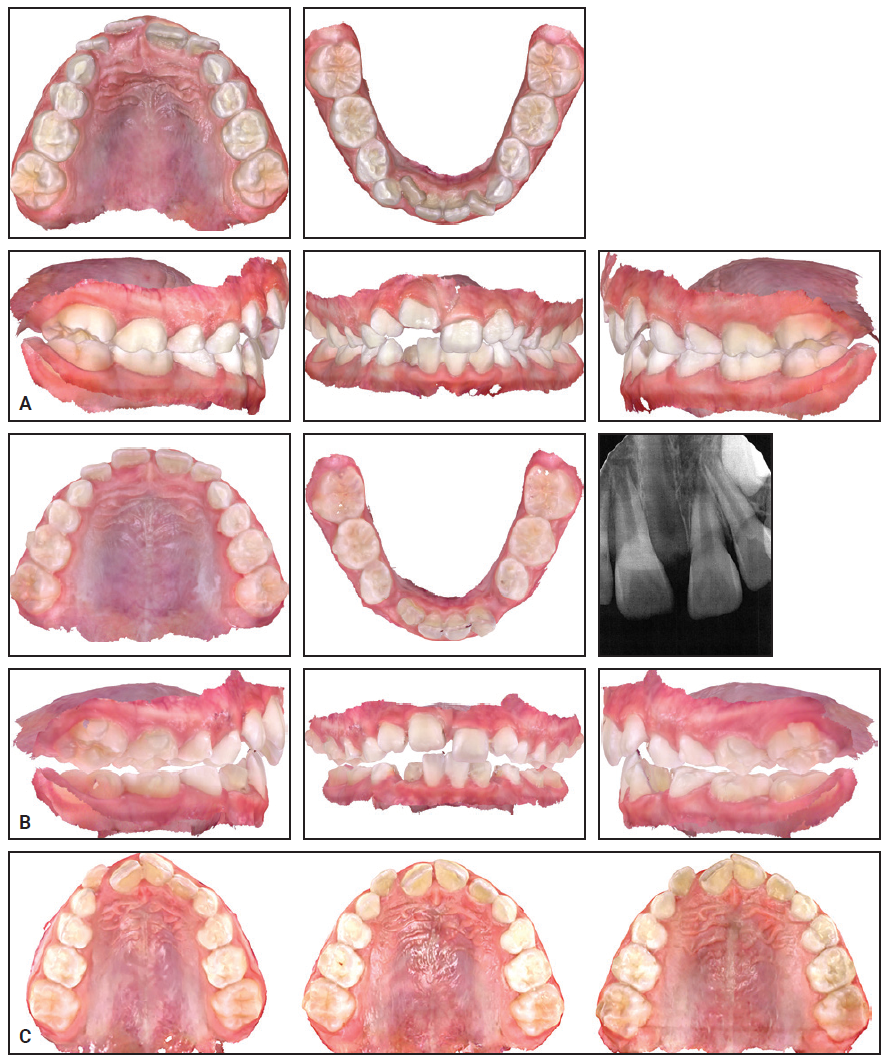
Fig. 5 Case 2. A. At beginning of treatment. B. After 32 of 34 prescribed stages of expansion with IPE. C. Progress of expansion: beginning of treatment (left), after expansion (center), and after four months of retention (right).
Conclusion
The ability to produce direct-printed, customized expanders by means of computer-aided design and manufacturing is a profound step forward for the orthodontic specialty. Align Technology’s IPE system applies decades of technological developments and improvements in digital workflows to enable efficient and effective treatment, with the potential to offer an improved patient experience and better clinical outcomes.
FOOTNOTES
- *Registered trademark of Align Technology, Inc., San Jose, CA; www.aligntech.com.
REFERENCES
- 1. Haas, A.J.: The treatment of maxillary deficiency by opening the midpalatal suture, Angle Orthod. 35:200-217, 1965.
- 2. Garib, D.G.; Henriques, J.F.C.; Janson, G.; de Freitas, M.R.; and Fernandes, A.Y.: Periodontal effects of rapid maxillary expansion with tooth-tissue-borne and tooth-borne expanders: A computed tomography evaluation, Am. J. Orthod. 129:749-758, 2006.
- 3. Garib, D.G.; Henriques, J.F.C.; Janson, G.; de Freitas, M.R.; and Coelho, R.A.: Rapid maxillary expansion—tooth tissue-borne versus tooth-borne expanders: A computed tomography evaluation of dentoskeletal effects, Angle Orthod. 75:548-557, 2005.
- 4. Maspero, C.; Cavagnetto, D.; Fama, A.; Giannini, L.; Galbiati, G.; and Farronato, M.: Hyrax versus transverse sagittal maxillary expander: An assessment of arch changes on dental casts: A retrospective study, Saudi Dent. J. 32:93-100, 2020.
- 5. Inchingolo, A.M.; Patano, A.; De Santis, M.; Del Vecchio, G.; Ferrante, L.; Morolla, R.; Pezzolla, C.; Sardano, R.; Dongiovanni, L.; Inchingolo, F.; Bordea, I.R.; Palermo, A.; Inchingolo, A.D.; and Dipalma, G.: Comparison of different types of palatal expanders: Scoping review, Children (Basel) 10:1258, 2023.
- 6. Rutili, V.; Mrakic, G.; Nieri, M.; Franceschi, D.; Pierleoni, F.; Giuntini, V.; and Franchi, L.: Dento-skeletal effects produced by rapid versus slow maxillary expansion using fixed jackscrew expanders: A systematic review and meta-analysis, Eur. J. Orthod. 43:301-312, 2021.




COMMENTS
.