CASE REPORT
Orthodontic Movement into a Bone Defect Augmented with a Heterologous Graft
Bony defects can present challenges in the orthodontic treatment of adult patients.1,2 Some studies have indicated that orthodontic movement into an intraosseous defect has no beneficial effect on tissue levels,3,4 while others show that attachment is possible with orthodontic movement into a site where tissue has been repaired.5,6 Depending on the amount of periodontal resorption, regenerative procedures to restore adequate bone volume before orthodontic movement can make attachment possible.7,8
This case report describes the hybrid treatment of a patient with a severe bone defect at the lower left second molar using a heterologous Bio-Oss* graft made from bovine bone matrix, followed by attempted orthodontic mesialization of the adjacent third molar.
Diagnosis and Treatment Plan
A 43-year-old male with the chief complaint of moderate crowding in the mandibular arch sought esthetic orthodontic treatment (Fig. 1). He presented a Class I skeletal pattern (ANB = 2°, Wits appraisal = –2mm) with bilateral Class I molar and canine relationships and a high-angle vertical pattern (GoGn-SN = 41°).
In the initial periapical x-rays, severe bone resorption was evident around the distal root of the lower left second molar, which had previously been treated with a rhizotomy of the mesial root. The lower left third molar showed a mesial bone peak without pathological findings on probing.9 Because of the extent of bone resorption, we decided to extract the lower left second molar and move the lower left third molar mesially into the extraction space. Regenerative procedures would be needed to restore the alveolar bone to a volume capable of receiving the mesialized third molar. The anterior crowding would be addressed with clear aligners** and interproximal reduction.
Similar articles from the archive:
- CASE REPORT Periodontal Regeneration and Orthodontic Intrusion of a Pathologically Migrated Central Incisor Adjacent to an Infrabony Defect July 2012
- Orthodontic Closure of a Midline Diastema with an Infrabony Defect March 2011
- CASE REPORT Treatment of an Isolated Vertical Infrabony Defect with Orthodontic Intrusion July 2009
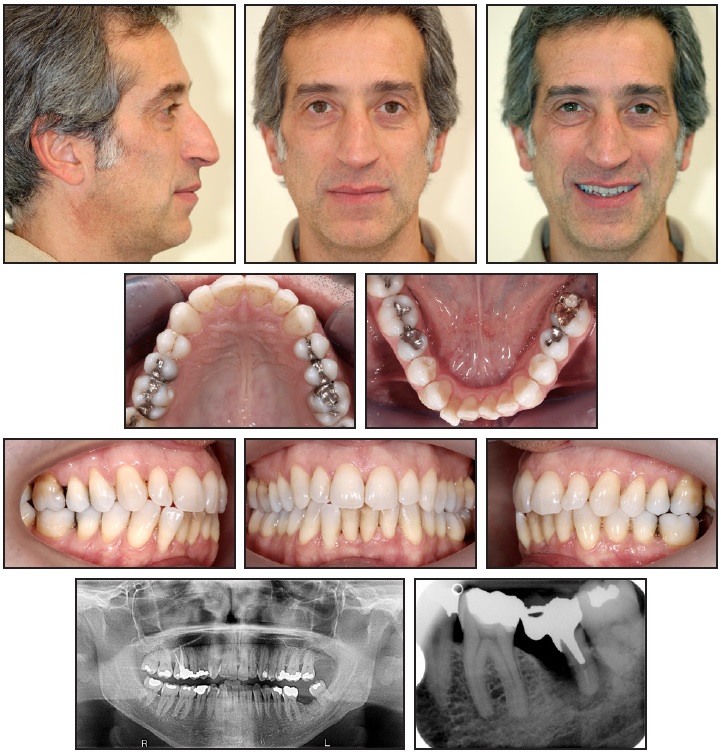
Fig. 1 43-year-old male patient with moderate lower crowding and severe bone resorption around distal root of lower left second molar before treatment.
Treatment Progress
During the surgical procedure for removal of the lower left second molar, a graft covered with resorbable membrane was applied. Eight months later, clear aligner therapy was initiated to align and coordinate the arches.10,11 A fixed orthodontic auxiliary, consisting of two .018" preadjusted brackets*** with a sectional .016" stainless steel wire and an elastomeric chain, was installed to close the space of the lower left second molar (Fig. 2). Anterior anchorage was provided by the aligners, which were cut off distal to the lower left first molar. The elastomeric chain was changed monthly.
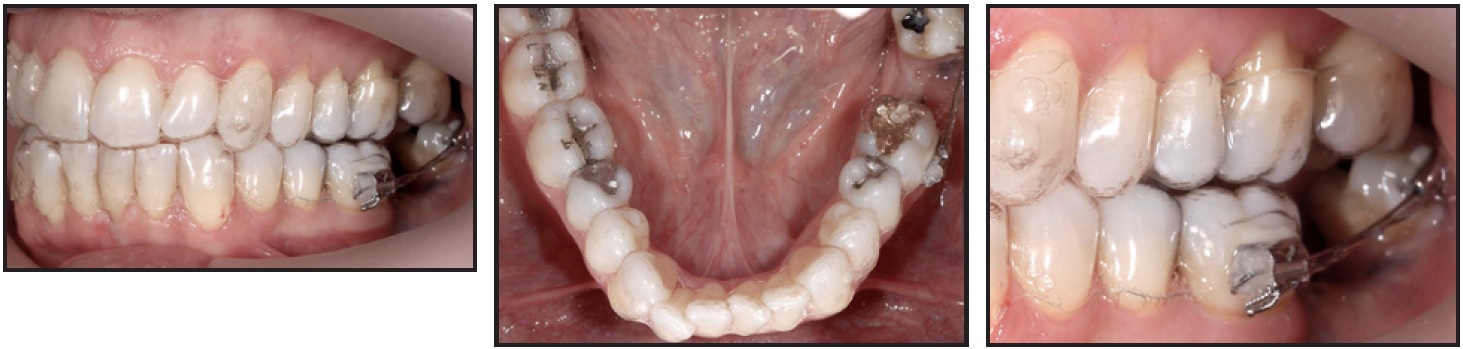
Fig. 2 Fixed auxiliary using two .018" brackets,*** .016" stainless steel wire, and elastomeric chain to mesialize lower left third molar, with clear aligner** serving as anterior anchorage.
After eight months, the anterior alignment had improved, but the lower left third molar had not moved sufficiently. The anchorage design and the mechanics were therefore modified (Fig. 3) to include splints from the lower right canine to the lower left canine (on the lingual side) and from the lower left canine to the lower left first molar (on the buccal side).
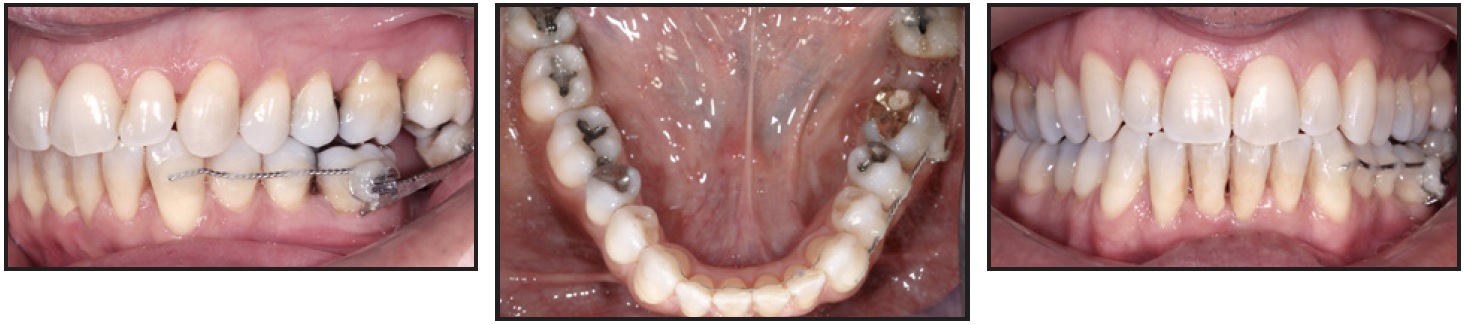
Fig. 3 Modified anchorage design after eight months of treatment, with splints added from lower right to lower left canine (on lingual side) and from lower left canine to lower left first molar (on buccal side).
Still, we observed that anchorage was being lost and the extraction space was not closing. The molar mesialization was stopped two months after placement of the modified auxiliary.
The x-ray taken at this point revealed that the graft had been an absolute obstacle to the movement of the lower left third molar, which showed severe signs of resorption on the mesial root adjacent to the grafted area (Fig. 4). The graft seemed to have acted in the same way as an implant ankylosed in the bone.
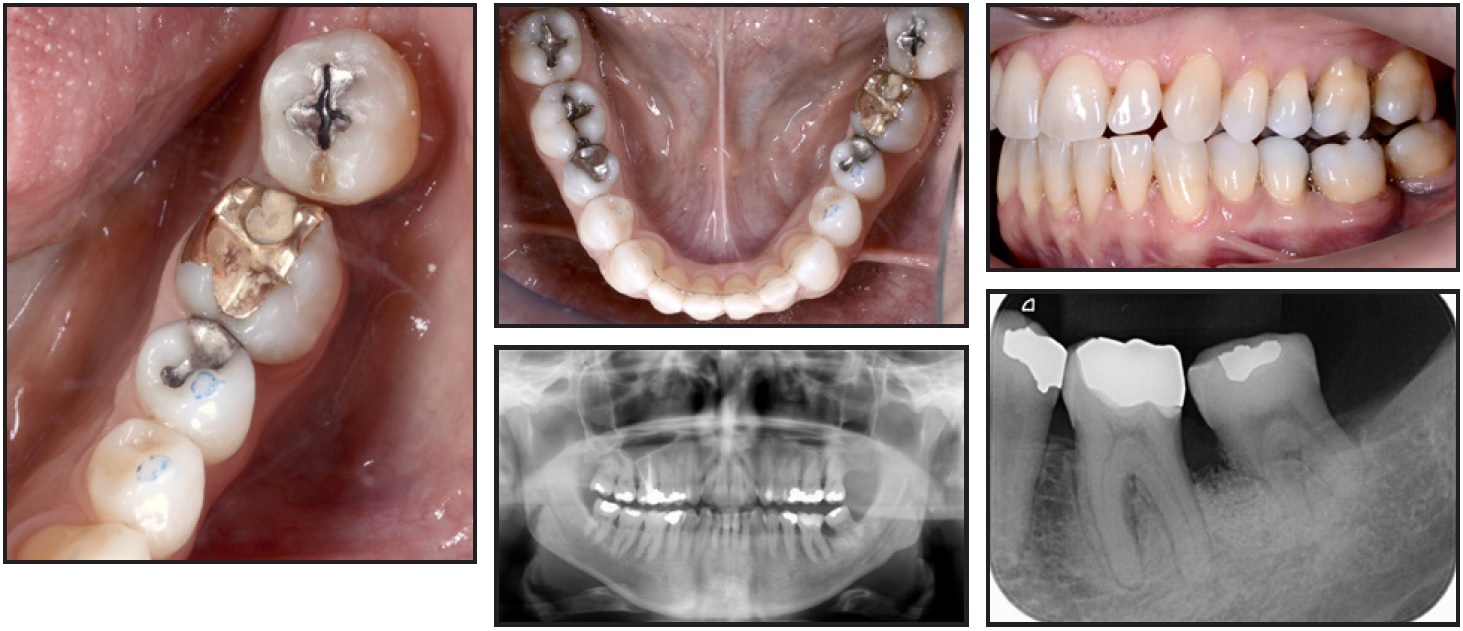
Fig. 4 Mesialization of lower left third molar stopped after 10 months of treatment, with graft acting as obstacle.
The vitality of the lower left third molar was monitored over time. Twenty-one months after the surgery, we noted a relapse of the loss of anchorage on the left side, with a slight improvement of the intercuspation. A composite build-up was then placed on the lower left first molar to create a contact point with the lower left third molar (Fig. 5).
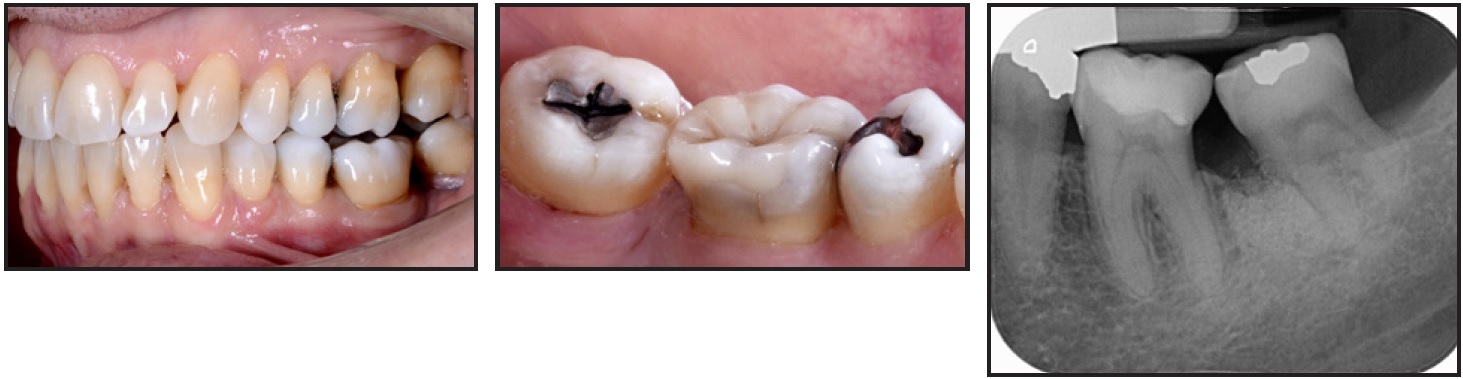
Fig. 5 After 21 months, composite build-up placed on lower left first molar to create contact point with third molar.
Treatment Results
Seven years later, the result was stable (Fig. 6). The lower left third molar maintained its pulp sensitivity during a cold test, and probing revealed no periodontal pathology.
Discussion
Among all the types of grafts reported in the literature,12 the only one with all the biological characteristics of osteoconductivity, osteogenicity, osteointegration, and osteoinductivity13-16 is the autologous graft.17-21 Disadvantages of this procedure are that it requires an additional surgery site, involves more chairtime, carries a greater risk of morbidity, and implies a higher biological cost. Other common types are heterologous grafts (as used in this case)12; homologous grafts, such as bank bone in fresh, frozen, demineralized, or cryopreserved form23,24; and biocompatible synthetic materials.12,25
Several authors have reported the possibility of directly moving teeth into postextraction alveoli and restoring adequate bone height, provided that light forces were used and adequate oral hygiene was maintained.26 Prior placement of a bone graft gives the alveolus better bone architecture and quality, however, with a lower risk of gingival recession.1,5
Hossain and colleagues analyzed orthodontic movement within autologous bone grafts (particulate marrow and cancellous bone) and beta-tricalcium phosphate ceramics in beagle dogs, finding less bone resorption and better biological response in the beta-tricalcium phosphate regions.27 Machibya and colleagues evaluated the behavior of xenografts (Bio-Oss) and alloplasts (beta-tricalcium phosphate) in beagles, observing favorable radiological characteristics (higher alveolar bone levels and bone density) in the Bio-Oss group, but faster tooth movement in the beta-tricalcium phosphate group.28 Bio-Oss grafting in beagles was also evaluated by Araújo and colleagues, who concluded not only that dental movement was possible, but also that the Bio-Oss had been reabsorbed in the areas affected by dental movement and had remained as inactive filler material in the other areas.29
Ahn and colleagues studied orthodontic movement at zero, two, and 12 weeks after surgery in beagles treated with alveolar osteotomy alone or osteotomy with a bone graft.30 The bone graft in the surgical defect allowed immediate force application for accelerated orthodontic tooth movement with favorable periodontal regeneration, while reducing the risk of inhibited tooth movement in cases of delayed force application after surgery.30 Oltremari and colleagues, in a study of minipigs, concluded that teeth can be moved into areas of bone defects previously filled with bovine bone matrix xenografts.31
The root resorption in the present case would not have been expected based on the studies listed above. In our patient, however, the orthodontic movement was initiated eight months after grafting—in contrast with the study by Ahn and colleagues, where it was delayed by a maximum of 12 weeks.30 Supporting this conclusion, Klein and colleagues, in a mouse study, found that the lack of resorption of bovine bone xenografts renders them inadequate for orthodontic tooth movement at a later stage.32 The same group of authors later published a study comparing allografts and beta-tricalcium phosphates, again in mice: both induced full, normal healing but hindered orthodontic tooth movement into the regenerated sites.33
There are few clinical reports of orthodontic tooth movement into grafted areas. Vitral and colleagues showed an enhancement of dental movement six months after autologous sinus grafting.34 Ruellas and colleagues35 and Nemcovsky and colleagues5 reported positive results from orthodontic movement within bone-bank allografts. In a study by Nagy and colleagues, histological evaluation showed greater bone neoformation in cases treated with deproteinized bovine-bone mineral grafts and early orthodontic movement, compared to those without orthodontic treatment, but the resorption of the grafts was only partial.36 Re and colleagues37 and Cardaropoli and colleagues38 presented cases in which early orthodontic movement (10-14 days) followed periodontal treatment with Bio-Oss and Tissucol,† respectively, producing no detrimental effects. Carvalho and colleagues described orthodontic movement into the region of a bone defect using an allograft with guided tissue regeneration and a nonresorbable membrane, but they emphasized that the type, magnitude, and clinical variability of the defect would determine the success of the procedure.39
As confirmed by our case, the time factor seems to be crucial in determining biological behavior and clinical results in these patients. We suggest the following guidelines:
- When movement of a single tooth through regenerated bone is planned, the use of a resorbable graft is preferable.
- When a nonresorbable graft is used, orthodontic movement should be started early.
FOOTNOTES
- *Geistlich Pharma AG, Wolhusen, Switzerland; www.geistlichpharma.com.
- **Invisalign, Align Technology, San Jose, CA; www.aligntech.com.
- ***American Orthodontics, Sheboygan, WI; www.americanortho.com.
- †Baxter AG, Opfikon, Switzerland; www.baxter.com.
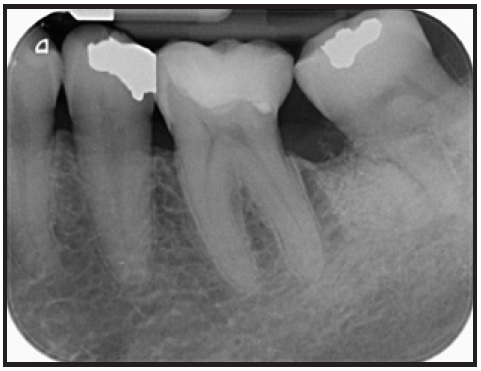
Fig. 6 Seven years after treatment.
REFERENCES
- 1. Diedrich, P.R.: Guided tissue regeneration associated with orthodontic therapy, Semin. Orthod. 2:39-45, 1996.
- 2. Pietrokovski, J.: The bony residual ridge in man, J. Prosth. Dent. 34:456-462, 1975.
- 3. Polson, A.; Caton, J.; Polson, A.P.; Nyman, S.; Novak, J.; and Reed, B.: Periodontal response after tooth movement into intrabony defects, J. Periodontol. 55:197-202, 1984.
- 4. Wennström, J.L.; Stokland, B.L.; Nyman, S.; and Thilander, B.: Periodontal tissue response to orthodontic movement of teeth with infrabony pockets, Am. J. Orthod. 103:313-319, 1993.
- 5. Nemcovsky, C.E.; Zubery, Y.; Artzi, Z.; and Lieberman, M.A.: Orthodontic tooth movement following guided tissue regeneration: Report of three cases, Int. J. Adult Orthod. Orthog. Surg. 11:347-355, 1996.
- 6. Diedrich, P.: The eleventh hour or where are our orthodontic limits? Case report, J. Orofac. Orthop. 60:60-65, 1999.
- 7. Rosa, M. and Ricci, C.: Orthodontics and periodontal therapy [in Italian], in Diagnosi e terapia parodontale, 3rd ed., ed. G. Ricci, Quintessenza Edizioni, Milan, 2012, p. 664.
- 8. Roberts, W.E. and Chase, D.C.: Kinetics of cell proliferation and migration associated with orthodontically-induced osteogenesis, J. Dent. Res. 60:174-181, 1981.
- 9. Calton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; and Tonetti, M.S.: A new classification scheme for periodontal and peri-implant diseases and conditions: Introduction and key changes from the 1999 classification, J. Clin. Periodontol. 45 Suppl. 20:S1-S8, 2018.
- 10. Boyd, R.L.; Oh, H.; Fallah, M.; and Vlaskalic, V.: An update on present and future considerations of aligners, J. Calif. Dent. Assoc. 34:793-805, 2006.
- 11. Hönn, M. and Göz, G.: A premolar extraction case using the Invisalign system, J. Orofac. Orthop. 67:385-394, 2006.
- 12. Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; and Gunsolley, J.C.: The efficacy of bone replacement grafts in the treatment of periodontal osseous defects: A systematic review, Ann. Periodontol. 8:227-265, 2003.
- 13. Khan, S.N.; Cammisa, F.P. Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; and Lane, J.M.: The biology of bone grafting, J. Am. Acad. Orthop. Surg. 13:77-86, 2005.
- 14. Kao, S.T. and Scott, D.D.: A review of bone substitutes, Oral Maxillofac. Surg. Clin. N. Am. 19:513-521, 2007.
- 15. Cypher, T.J. and Grossman, J.P.: Biological principles of bone graft healing, J. Foot Ankle Surg. 35:413-417, 1996.
- 16. Precheur, H.V.: Bone graft materials, Dent. Clin. N. Am. 51:729-746, 2007.
- 17. Jäger, M.; Westhoff, B.; Wild, A.; and Krauspe, R.: Bone harvesting from the iliac crest [in German], Orthop. 34:976-982, 984, 986-990, 992-994 passim, 2005.
- 18. Misch, C.M.: Comparison of intraoral donor sites for onlay grafting prior to implant placement, Int. J. Oral Maxillofac. Implants 12:767-776, 1997.
- 19. Boyne, P.J. and Sands, N.R.: Secondary bone grafting of residual alveolar and palatal clefts, J. Oral Surg. 30:87-92, 1972.
- 20. McAllister, B.S. and Haghighat, K.: Bone augmentation techniques, J. Periodontol. 78:377-396, 2007.
- 21. Bavetta, G. and Licata, M.E.: The use of human allogenic graft (HBA) for maxillary bone regeneration: Review of literature and case reports, Curr. Pharm. Des. 18:5559-5568, 2012.
- 22. Gazdag, A.R.; Lane, J.M.; Glaser, D.; and Forster, R.A.: Alternatives to autogenous bone graft: Efficacy and indications, J. Am. Acad. Orthop. Surg. 3:1-8, 1995.
- 23. Perrott, D.H.; Smith, R.A.; and Kaban, L.B.: The use of fresh frozen allogeneic bone for maxillary and mandibular reconstruction, Int. J. Oral Maxillofac. Surg. 21:260-265, 1992.
- 24. Dallari, D.; Fini, M.; Stagni, C.; Torricelli, P.; Aldini, N.N.; Giavaresi, G.; Cenni, E.; Baldini, N.; Cenacchi, A.; Bassi, A.; Giardino, R.; Fornasari, P.M.; and Giunti, A.: In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination, J. Orthop. Res. 24:877-888, 2006.
- 25. Garrett, S.: Consensus report: Periodontal regeneration around natural teeth, Ann. Periodontol. 1:667-670, 1996.
- 26. Thilander, B.: Infrabony pockets and reduced alveolar bone height in relation to orthodontic therapy, Semin. Orthod. 2:55-61, 1996.
- 27. Hossain, M.Z.; Kyomen, S.; and Tanne, K.: Biologic responses of autogenous bone and beta-tricalcium phosphate ceramics transplanted into bone defects to orthodontic forces, Cleft Palate Craniofac. J. 33:277-283, 1996.
- 28. Machibya, F.M.; Zhuang, Y.; Guo, W.; You, D.; Lin, S.; Wu, D.; and Chen, J.: Effects of bone regeneration materials and tooth movement timing on canine experimental orthodontic treatment, Angle Orthod. 88:171-178, 2018.
- 29. Araújo, M.G.; Carmagnola, D.; Berglundh, T.; Thilander, B.; and Lindhe, J.: Orthodontic movement in bone defects augmented with Bio-Oss: An experimental study in dogs, J. Clin. Periodontol. 28:73-80, 2001.
- 30. Ahn, H.W.; Ohe, J.Y.; Lee, S.H.; Park, Y.G.; and Kim, S.J.: Timing of force application affects the rate of tooth movement into surgical alveolar defects with grafts in beagles, Am. J. Orthod. 145:486-495, 2014.
- 31. Oltramari, P.V.P.; De Lima Navarro, R.; Henriques, J.F.C.; Taga, R.; Cestari, T.M.; Ceolin, D.S.; Janson, G.; and Granjeiro, J.M.: Orthodontic movement in bone defects filled with xenogenic graft: An experimental study in minipigs, Am. J. Orthod. 131:302e10-302e17, 2007.
- 32. Klein, Y.; Fleissig, O.; Stabholz, A.; Chaushu, S.; and Polak, D.: Bone regeneration with bovine bone impairs orthodontic tooth movement despite proper osseous wound healing in a novel mouse model, J. Periodontol. 90:189-199, 2019.
- 33. Klein, Y.; Kunthawong, N.; Fleissig, O.; Casap, N.; Polak, D.; and Chaushu, S.: The impact of alloplast and allograft on bone homeostasis: Orthodontic tooth movement into regenerated bone, J. Periodontol. 91:1067-1075, 2020.
- 34. Vitral, R.W.F; Da Silva Campos, M.J.; De Andrade Vitral, J.C.; Santiago, R.C.; and Fraga, M.R.: Orthodontic distalization with rigid plate fixation for anchorage after bone grafting and maxillary sinus lifting, Am. J. Orthod. 136:109-114, 2009.
- 35. Ruellas, A.C.O.; Cunha, A.C.; Valadares, C.V.; Tenório De Sá, A.P.; and Ruellas, C.V.O.: Orthodontic movement of posterior teeth into a corticocancellous bone-block allograft area, J. Clin. Orthod. 50:427-436, 2016.
- 36. Nagy, P.; Molnar, B.; Nemes, B.; Schupbach, P.; and Windisch, P.: Histologic evaluation of human intrabony periodontal defects treated with deproteinized bovine bone mineral in combination with orthodontic tooth movement: A case series, Int. J. Period. Rest. Dent. 40:321-330, 2020.
- 37. Re, S.; Corrente, G.; Abundo, R.; and Cardaropoli, D.: Orthodontic movement into bone defects augmented with bovine bone mineral and fibrin sealer: A reentry case report, Int. J. Period. Rest. Dent. 22:138-145, 2002.
- 38. Cardaropoli, D.; Re, S.; Manuzzi, W.; Gaveglio, L.; and Cardaropoli, G.: Bio-Oss collagen and orthodontic movement for the treatment of infrabony defects in the esthetic zone, Int. J. Period. Rest. Dent. 26:553-559, 2006.
- 39. Carvalho, R.S.; Nelson, D.; Kelderman, H.; and Wise, R.: Guided bone regeneration to repair an osseous defect, Am. J. Orthod. 123:455-467, 2003.






COMMENTS
.