Think back to your most stressful and exhausting finals week (for me it was my second year in dental school—14 finals in five days). Recall how you felt after surviving on caffeine and adrenaline and no sleep: irritable, physically tired, mentally fuzzy, short-tempered, and unfocused. When it was over, it took a few days or even weeks to catch up on sleep and rejuvenate.
Now, imagine that you never recover. Every time you enter deep sleep, your breathing stops and you begin to suffocate. Eventually, your body releases adrenaline and jars you back to a light sleep. Even working harder to breathe through snoring or upper-airway resistance results in many of the same comorbidities as full-blown obstructive sleep apnea (OSA). This cycle is repeated, sometimes 50-100 times per hour, every night, forever. Living with OSA is like reliving the end of your worst finals week—forever.
It doesn’t happen overnight. The symptoms gradually increase over time, so you don’t fully realize what you’ve lost. The lack of deep sleep and long-term oxygen deprivation lead to an increased risk of type 2 diabetes, high blood pressure, cardiac arrest, Alzheimer’s disease, and stroke. OSA affects nearly every system in the body—from neurological to hormonal to immunological. The hormonal changes increase your appetite for fast-energy foods and depress your ability to realize you’re full, so that OSA often leads to morbid obesity. I know this personally.
I was first diagnosed with OSA in the mid-2000s. By then, I had my master’s degree in orthodontics and my wife, Penny, was a nurse practitioner in neurology. Despite our training, it took years to realize that my mental and social difficulties were not just me being a jerk, but were actually due to a lack of deep sleep. It wasn’t until Penny was awake all night, poking me every time I stopped breathing, that we sought help. I had a series of polysomnograms (PSGs) and was diagnosed and fitted for a continuous positive airway pressure (CPAP) machine. It was (and still is) the first treatment recommended for OSA. At that point, I reasoned that since this disease manifests as a collapse of the airway, there must be an anatomical component, and since we deal with facial and dental anatomy every day, orthodontists may have some solutions to offer. Since then, I have been on a professional and personal journey to help understand, screen for, and treat this disease.
OSA Is Prevalent
How does OSA affect orthodontic practices? First, realize that an estimated 20% of the adults in the United States (about 18 million) have OSA, but about 85% of those are undiagnosed.1-4 Forty percent of men and 24% of women snore; although OSA worsens with age and increased weight,1-4 it isn’t just a disease of overweight, middle-age men. In 2009, an estimated 10% of children snored regularly, and 2-4% suffered from OSA.1-4 I suspect those numbers have risen since then. The symptoms in children are often different—less daytime sleepiness and more hyperactivity,5 behavioral problems, and drops in intelligence scores.6 The potential long-term effects of sleep and oxygen deprivation during the years in which the face and intellect are developing are daunting. The bottom line is that our towns and practices are filled with people who have undiagnosed breathing and airway issues. We can help.
Medical Diagnosis
OSA is one of hundreds of sleep disorders, which means that its diagnosis is a medical responsibility. This is not to say that orthodontists can’t or even shouldn’t have a partial role—just that the primary diagnosis is made by a medical doctor, and the initial treatment is generally rendered by the medical community.
In general, the process goes as follows: symptoms are identified, and the patient is referred for evaluation to a sleep doctor, who provides a home sleep test to wear overnight. If the results of the test are positive or potentially a false negative, an attended overnight sleep test (PSG) is ordered. In a PSG, the patient is connected to several dozen leads taking 14-20 physiological measurements, including brain activity (EKG), muscle activity (EMG), blood pressure, and clenching. The results are then evaluated by a board-certified sleep physician.
The results of a PSG are reported using the apnea-hypopnea index (AHI) or respiratory disturbance index (RDI), along with oxygen desaturation levels. An apnea is defined as an instance when the patient stops breathing for 10 seconds or more. A hypopnea is when the patient does not stop breathing, but exhibits shallow breathing (a partial obstruction) that results in a blood oxygen drop of 3-4%. The AHI is the total number of apneas and hypopneas per hour.
Respiratory event-related arousals (RERAs) have been shown to have similar comorbidities to apneas and hypopneas. An RERA is characterized by increased respiratory effort for 10 seconds or more, leading to an arousal from sleep that does not fulfill the criteria for apneas or hypopneas. The RDI is the total number of apneas, hypopneas, and RERAs per hour.
In adults, an AHI or RDI of less than 5 is considered normal; 5-15 indicates mild OSA, 16-30 is moderate, and greater than 30 is severe. In children, an AHI or RDI greater than 1 is considered abnormal and should be treated.7
Blood oxygen level is measured as a percentage, with 95% or more considered normal. Blood oxygen levels vary slightly throughout the day, but a level below 90% is a cause for concern. In fact, it is unusual for the oxygen level in an awake patient to drop below 94%. During apnea, oxygen desaturation can drop into the 80s or even 70s, generally resulting in an arousal in which the body senses the drop and forces itself out of deep sleep to address the low oxygen level.
Screening
Like most professionals, orthodontists tend to notice and treat things we know we have solutions for. I personally did not notice tongue ties until I learned how to relieve them with my soft-tissue laser. I became familiar with the signs and symptoms of unhealthy breathing when I found that I had solutions for some of these issues. My office now screens every patient (and family member) for OSA and diminished airway. That meant changing a number of things in our diagnostic protocol.
First, we added a few questions to our health history:
- Have you ever been diagnosed with sleep apnea?
- Do you snore?
- Do you have headaches when you wake up?
- Have you ever worn a CPAP?
- Are you aware or have you been told that you stop breathing during sleep?
For children—who exhibit different behaviors with OSA—we added these questions for their parents to answer:
- Does your child have ADD or ADHD? [While only an estimated 2-3% of children have OSA, 25-26% of children with even mild attention-deficit/hyperactivity disorder (ADHD) have OSA.5]
- Does your child snore or “breathe heavily” while sleeping? [Children should never snore; the American Academy of Pediatrics issued a statement in 2012 that all children should be screened for breathing problems, and that any who snore should be tested for sleep-disordered breathing (SDB) and sleep apnea.8]
- Do they wet the bed?
- Do they wake up in the same position or are they “all over the bed” at night?
- Is there increased sweating?
- Any changes in behavior or difficulty “managing” themselves at school?
- Any marked changes in the child’s attention? [Johns Hopkins found the average IQ in a group of children with OSA to be 85, as compared with 101 in a matched non-OSA group.6]
Second, we pay particular attention to aspects of the health history that are associated with OSA, including a family history of high blood pressure, type 2 diabetes, cardiac arrest, stroke, obesity, gastroesophageal reflux disease (GERD), or Alzheimer’s disease.
Third, every patient fills out a sleep questionnaire. We ask adults to fill out the Epworth Sleepiness Scale; for children, we use the Pediatric Sleep Questionnaire from the University of Michigan.
Next, I pay particular attention to aspects of our extra- and intraoral examination that are associated with OSA. Some examples:
1. Head posture: The skull should sit over the shoulders. When there is difficulty breathing, it is not uncommon to see hyperextension of the neck, resulting in a forward head posture, or kyphosis. This can be detected as the patient enters the room.
2. Adenoid facies: Also known as long-face syndrome, adenoid facies was first described by C.V. Tomes in 1872.9 As orthodontists, we are well aware of the signs of adenoid facies, many of which are associated with SDB.
3. Nasal anatomy: Since an obstruction can occur anywhere along the upper airway, I’ve added the size and shape of the nares, nasal asymmetries and deviations, and a number of radiographic items to my exam. Narrow, collapsible nares or intranasal inflammation can negatively affect the upper airway. Likewise, a severe septal deviation can reduce airflow.10-12
4. Skeletal retrognathia: This condition can negatively affect the airway. It is not uncommon to see a retrognathic mandible accompanying a dental overjet or Class II, division 1 malocclusion. It is often more difficult to recognize, however, when there is a Class I occlusion with no overjet, and both the upper and lower jaws are retrognathic. Ideally, there should be some prominence to the cheekbones; flat or concave cheekbones may indicate a maxillary anterior deficit.
5. Neck size: One of the indicators for sleep apnea is a neck circumference greater than 17" for males or 16" for females.13
6. Tongue tie: It has often been noted that mouthbreathers tend to exhibit excessive vertical growth, which has been attributed in part to the tongue not staying in the roof of the mouth during swallowing. One underdiagnosed symptom is ankyloglossia, where the tongue cannot reach the roof of the mouth. This has long been suspected as a contributor to OSA, and recent studies have confirmed the relationship.14,15
7. Enlarged tonsils/adenoids: Although they are easily diagnosed from cone-beam computed tomography (CBCT), enlarged adenoids can usually be seen on a lateral cephalogram as well, and palatine tonsils can often be seen during intraoral examination.14 The tonsillar grading scale is used to indicate the potential severity of the impact of palatine tonsils on the airway.16
8. GERD: A known side effect of OSA,17 GERD presents as smooth, mother-of-pearl-like depressions on the occlusal or lingual surfaces of the teeth (Fig. 1).
9. Clenching/grinding: Sleep bruxism occurs in 2-40% of the pediatric population18 and 8-16% of the adult population.18,19 When you note clenching or grinding in the exam, look closely for other signs and symptoms of OSA.
Radiography
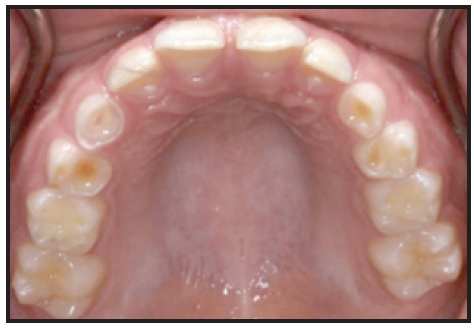
Fig. 1 8-year-old male showing dental depressions characteristic of gastroesophageal reflux disease.
As viewed on CBCT, the minimal cross-sectional area (MCA) of the airway when prone and sleeping is about 40% of the MCA when upright and awake.20 The average MCA for non-OSA adults is about 150mm2, but with a large standard deviation.21 Avrahami and Englender have suggested that patients are at low risk for OSA if the MCA is greater than 110mm2, at moderate risk if the MCA is 50-110mm2, and at high risk if the MCA is less than 50mm2.22
Schendel and colleagues analyzed the airways of 1,300 subjects ranging in age from 6 to 90.23 Their results have been used to indicate that a normal MCA during childhood is about 10 times the subject’s age. While these figures are roughly correct, they are based on averages with substantial standard deviations (Table 1).
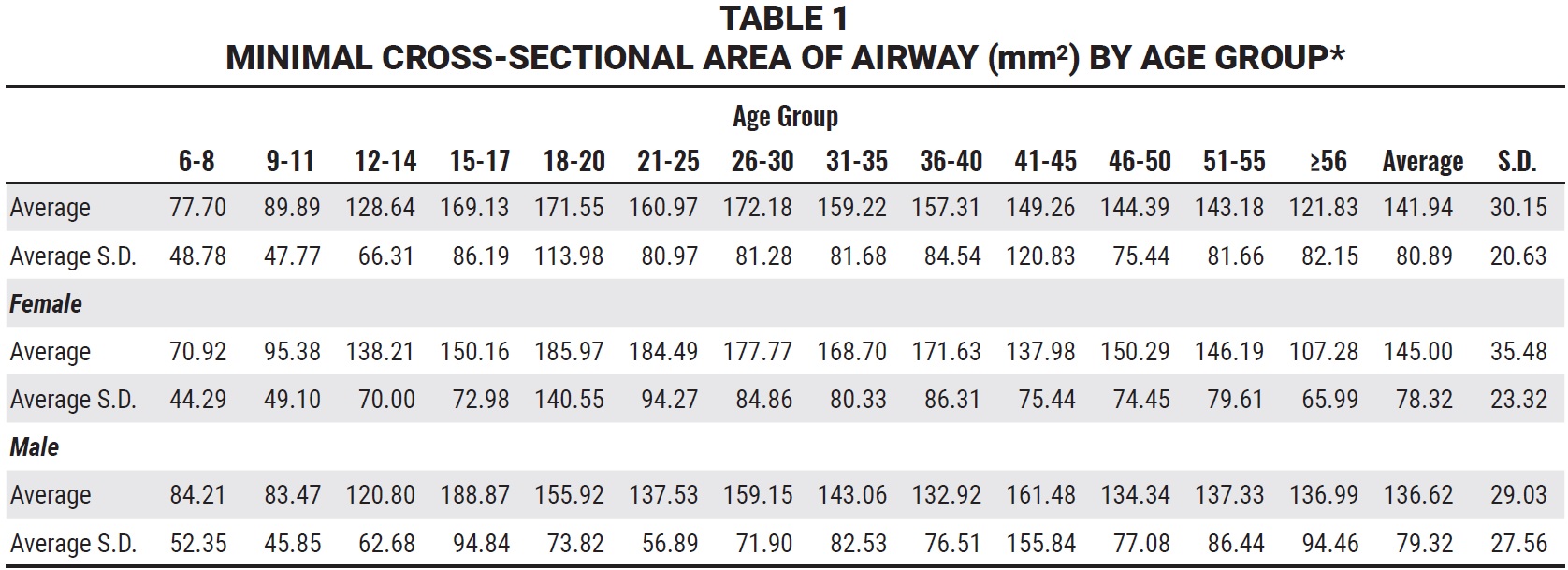
Since it is rare that an orthodontic patient would be exactly average, the authors’ conclusion seems appropriate: “The airway size and length increases until age 20, at which time there is a variable period of stability, after which the airway at first decreases slowly in size and then, after age 50, more rapidly” (Fig. 2).22 Therefore, we want to maximize the airway before age 20.
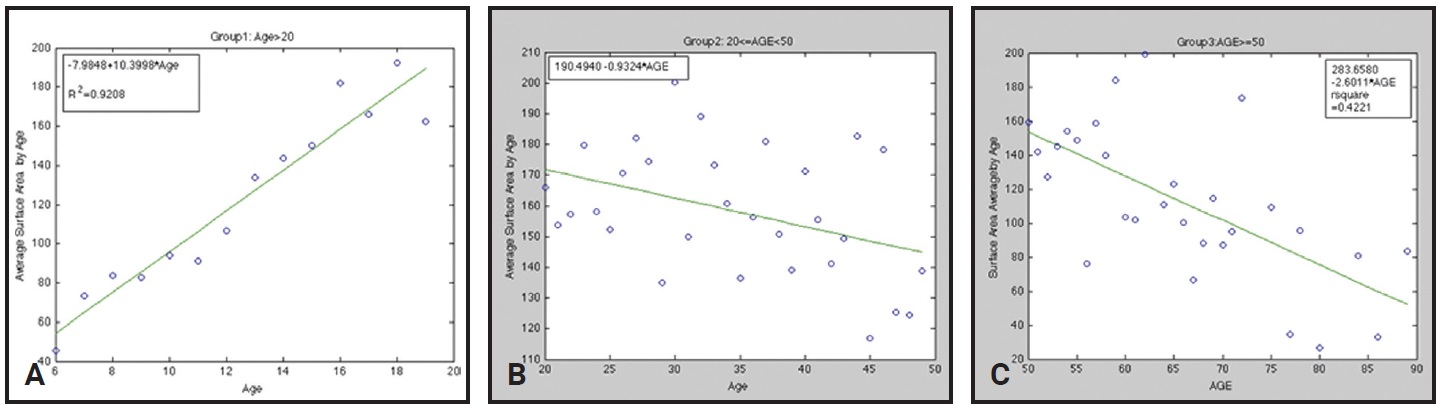
Fig. 2 Minimal cross-sectional areas of airways in different age groups. A. Group 1: ages 6-20. B. Group 2: ages 21-50. C. Group 3: ages 51-90.*
There is a large variability in MCA during exhaling, inhaling, and swallowing, making it a poor predictor of OSA or breathing difficulties and of questionable use in comparing the efficacy of various treatment modalities (Fig. 3).
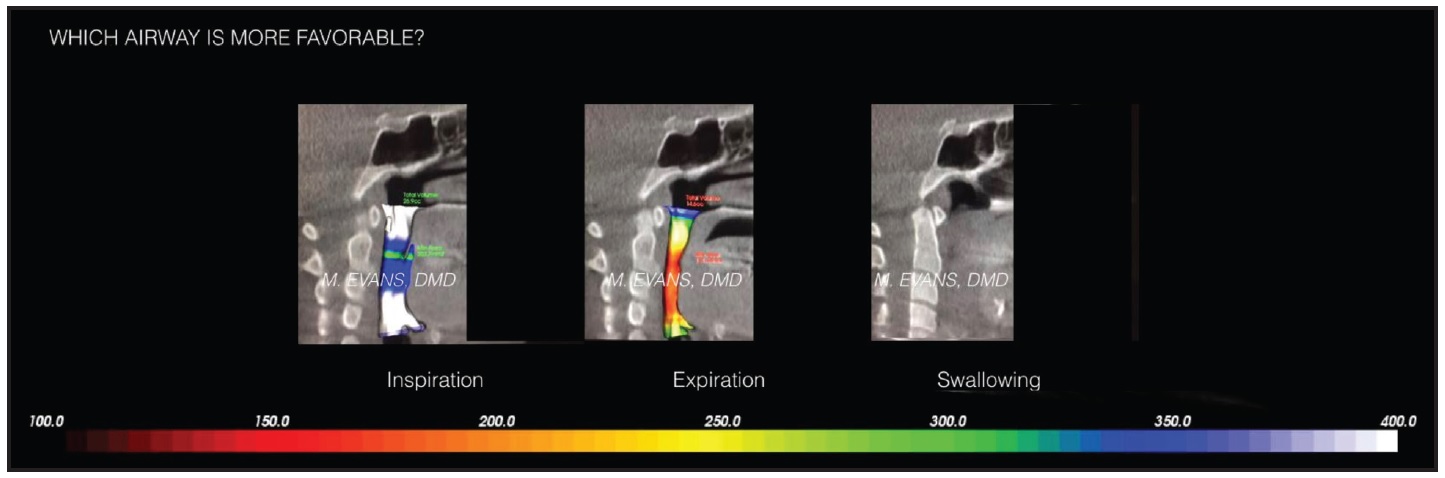
Fig. 3 Cone-beam computed tomography showing variability of airway measurements during inspiration, expiration, and swallowing (courtesy of Dr. Marianna Evans).
To account for these differences, Iwasaki and colleagues attempted to standardize the patient’s position while taking CBCT scans.24 When we attempted to replicate their method, we found that when the patients held their breath, they also occluded the airways with their tongues, so that the obstructions increased to 100%.
This is not to say that CBCT does not have a place in OSA/airway diagnosis and treatment. It allows us to more precisely measure the relative width of the jaws, helps us determine the location of an obstruction, and gives us a more accurate way to superimpose volumes and evaluate treatment effects.
It’s More than Anatomy
OSA has components that involve not only aberrant anatomy, but also physiological and behavioral aspects.25 Treating OSA is truly interdisciplinary! Although one modality of treatment will rarely solve all the underlying causes of OSA or another breathing disorder, orthodontic treatment is certainly one aspect of this important care. Therefore, it is instructional to consider the differences between orthodontic and medical treatment goals.
Orthodontists strive for perfection: perfect alignment, ideal smile arc, facial harmony. In medicine, you can’t always achieve perfection, and any movement toward the goal is considered progress—for example, reducing the severity of OSA, even if it isn’t fully eliminated.
Orthodontists tend to solve most of the problems we see single-handedly. When we think of interdisciplinary care, we’re primarily thinking of aligning teeth before restorations. In OSA treatment, we need to interact with a plethora of other professional specialists, including board-certified sleep doctors, pulmonologists, ENTs, myofunctional therapists, cardiologists, and pediatricians.
In orthodontics, we tend to focus on biomechanics to solve problems. OSA is a multifactorial disorder that affects nearly every bodily system. With a breathing disorder, it is critical not only to correct the anatomy, but also to train the patient to change habits and to work with the medical community to alter physiology.
Behavioral Treatments
There are a host of strategies requiring only patient cooperation to help reduce the severity of OSA. These should be mentioned in the course of advising patients with OSA:
- Reduce alcohol intake, particularly after 6 p.m.
- Quit smoking.
- Avoid caffeine and other stimulants after noon.
- Minimize exposure to blue-light screens for at least two hours before sleeping.
- Reduce weight. Because OSA is exacerbated by weight gain, losing weight often reduces severity. The difficulty with this advice is that hormonal changes accompanying OSA may include reduced leptin,26,27 which signals fullness, and increased ghrelin, which stimulates caloric intake. Those who suffer from OSA thus have an increased appetite for food and, at the same time, less ability to recognize when they are full.27
Eliminating these behaviors, which are often significant contributors to OSA, can be difficult for sleep-deprived patients. There are alternatives.
CPAP
In an adult with a confirmed OSA diagnosis, CPAP is the standard first treatment. While there are many variations in equipment, a CPAP machine basically involves a mask held on by straps around the head and neck, connected to a fan that forces air through the upper airway into the lungs. A post-diagnostic PSG is usually required to determine the proper air pressure needed to push past the obstruction.
CPAP has been shown to be as much as 95% effective in normalizing AHI when worn properly.28 Due to difficulties maintaining a seal, skin irritation, claustrophobia, headaches, and dehydration, however, noncompliance is greater than 70%, often rendering CPAP ineffective as a long-term solution.29 There is also evidence that long-term use can disrupt cellular immune factors and increase the risk of upper respiratory and sinus infections.30
In a growing child, a CPAP machine can have significant negative social effects, and the backward pressure of the mask may limit anterior growth of the maxilla and mandible, which is anatomically contrary to the preferred direction of growth.31 Perhaps most important, CPAP treatment does not correct the underlying anatomy—the patient must wear it indefinitely for it to be effective.
Other Medical Solutions
- Tracheotomy: nearly 100% effective, but considered a last resort.
- Uvulopalatopharyngoplasty: about 50% effective.32
- Somnoplasty: efficacy under evaluation.
- Genioglossus advancement: to bring the tongue forward, a rectangular section of chin bone, where the genioglossus muscle attaches, is advanced, rotated 90°, and reattached.
- Inspire**: a programmable neurostimulator with breathing sensors and leads attached to the tongue is implanted in the patient’s chest and controlled by a remote. The patient turns the device on while sleeping, and it activates the tongue.
Oral Appliances
The American Academy of Sleep Medicine recommends oral appliances as an alternative to CPAP in cases of mild to moderate sleep apnea, or when there is noncompliance with CPAP. While they are only 70% effective for treatment of sleep apnea, oral appliances are easier to wear than CPAP devices, so that compliance is often higher. It is difficult to compare the efficacy of oral appliances to CPAP because the criteria for success are different. With CPAP, the goal is to reduce AHI into the normal range. An oral appliance is considered successful if the AHI is reduced by 50%, which means that a severe case of OSA may persist.33 Another limitation of oral appliances is that they do not correct the underlying anatomy.33
There are hundreds of oral appliance designs, but the majority function by bringing the mandible, and therefore the tongue, forward and out of the pharyngeal airway. There is also evidence that increasing the vertical dimension can improve airflow with less mandibular protrusion, provided the lips can still close. It should be noted that while CPAP pressures can be increased to bypass obstructions anywhere in the upper airway, an oral appliance works best when the obstruction is in the pharyngeal airway.
In addition, the more forward the mandible is positioned, the more likely the patient will experience muscle soreness, jaw pain, or tooth movement. Some of these side effects can be reduced by trading vertical for anterior positioning.
Orthodontic Treatment
There is no one-size-fits-all solution to sleep apnea; treatment generally requires not only more than one approach, but often more than one specialty.34 Whether combining orthodontic alignment with orthognathic surgery to advance both jaws or combining a sleep appliance with CPAP to reduce the needed pressures and improve the seal, we must think beyond our own offices and learn to partner with other professionals.
When a patient is diagnosed with OSA, I consider the following:
- Diagnosing the location of the obstruction will allow an optimal strategy to be designed.
- Treating patients with sleep apnea is about much more than offering sleep appliances. It’s about including anatomical and behavioral improvements in the diagnosis and treatment planning, to effect lasting change that doesn’t require patients to wear something whenever they sleep.
- Bringing the mandible forward is often advantageous.
- Increasing the vertical dimension is often advantageous.
- Increasing maxillary width and uprighting the lower posterior teeth have been proven to be advantageous.
- Nasal breathing is preferred.
There are a number of benefits to nasal breathing. First, the inspired air is filtered by the nasal hairs to reduce the amount of pollen, dust, or other allergens causing inflammation that reaches the upper airway. Second, nasal breathing allows the air to be humidified and warmed, enhancing the transfer of O2 and CO2 in the lungs. Finally, during nasal breathing, nitric oxide is released. Nitric oxide is a vasodilator, which enhances blood flow, leading to a more efficient transfer of O2 to the rest of the body.
Nasal breathing also seems to have positive effects on facial growth.35 Alves and colleagues, comparing the airway dimensions between groups of mouthbreathers and nasal breathers, found that the nasal breathers had greater airway volumes and MCAs and thus greater airflow.36 Hakan and colleagues, comparing the airway volumes of 140 boys and girls grouped by molar classification, observed that the Class II subjects had significantly lower volumes of both the oropharynx and the nasopharynx than in the other groups.37
The Anteroposterior Dimension
Oral appliances move the mandible forward and improve AHI in most cases. Orthognathic surgery brings the mandible (or both jaws) forward, resulting in a dramatic reduction in AHI. Clearly, from an OSA standpoint, it is advantageous to bring the jaws forward.
The problem is that because orthodontic treatments have limited potential for altering mandibular growth,38 most techniques involve more mandibular anterior positioning than true growth. In a patient with OSA, it may not be enough to allow the mandible to position forward, since the worst AHI scores tend to occur during REM sleep, when voluntary muscles are paralyzed. This is an example of where a combination of approaches may be necessary: allowing the mandible to position forward, but perhaps also using an appliance while sleeping to hold it forward and keep the bite closed.
When the mandible is shorter than normal, there is often a concomitant relative narrowness of the maxilla (so that a wider part of the mandible fits against a wider part of the maxilla). McNamara and others described a “spontaneous” Class II correction after expansion of the maxilla,39,40 but this is debatable. Haas showed that correcting incisor angulation in a Class II, division 2 patient often results in forward positioning of the mandible.41-43
One of the more difficult diagnoses to make is when both the maxilla and the mandible are retrognathic. In this case, there may be a Class I occlusion with normal overbite and overjet, but impingement of the airway due to jaw position. Although it has been suggested that activating the premaxillary suture by pushing the upper incisors forward is the same as maxillary advancement,44 many of the presented examples seem to show more tipping of the upper incisors than actual jaw growth.
The Vertical Dimension
Less studied are changes in the vertical dimension and their effect on OSA. Again, some of the evidence comes from the oral appliance literature. When a mandibular-advancement appliance is fabricated to treat sleep apnea, a protrusive bite is used. In our office, we use an Andra Gauge*** because it allows adjustment of the vertical dimension in addition to the anteroposterior and lateral dimensions. A video illustrates what is called the “Step Back Technique,” in which the patient’s mandible is first brought forward until they cannot make a snoring sound, then the orthodontist “trades” a millimeter of protrusion at a time for a millimeter of increased vertical dimension. This is important because the more protrusion, the more anteroposterior force on the teeth, and therefore the more tooth movement from an oral appliance. The increase in the vertical dimension is limited by the necessity to keep the lips closed. Having made about 100 of these appliances with the Andra Gauge, I can attest that we can, in fact, trade protrusion for vertical.
In addition, we have numerous case reports of Class III patients who wore elastics to Class III Carriere Motion† appliances, which cause retraction and extrusion of the lower canines and thus open the bite. In our office, all such patients have shown increases in MCA, and many report fewer SDB symptoms.
The Transverse Dimension
This is the best-studied aspect of orthodontic care with respect to OSA. It has led, however, to a major controversy over which is better: expansion or extraction?
Let’s start with diagnosis. Traditionally, the need for expansion was determined by the presence of a dental crossbite or a “high, vaulted palate.” Miner and colleagues showed the problem with such a limited view: the angulation of the posterior teeth may mask an underlying skeletal discrepancy45 (Fig. 4). They compared the relative widths of the upper and lower jaws, dividing them into five groups based on the angulation of the posterior teeth. The subjects with no crossbites and minimal curves of Wilson (33% of the total sample) had relative widths similar to those of the inferior convergent group (20%), with both upper and lower posterior segments tipped lingually. The remaining 47%, including unilateral crossbite, bilateral crossbite, and superior convergent groups, also had similar relative widths. The authors concluded that there was “significant maxillary narrowness even in the absence of crossbite.”45
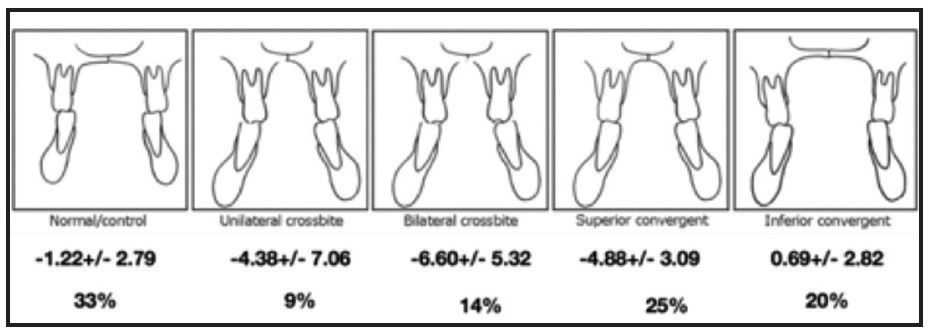
Fig. 4 Relative widths of five groups of upper and lower jaws, divided according to angulation of posterior teeth.‡
McNamara suggested that a “transpalatal width of 36-39mm would accommodate a dentition of average size without spacing or crowding, whereas maxillary arches less than 31mm in width may be crowded and in need of orthopedic or surgically assisted expansion.”46 The “transpalatal width” is measured as the distance between the closest points of the upper first molars. Besides failing to account for tipping of teeth, however, this method uses tooth positions to determine jaw discrepancies, and it is counterintuitive: it’s hard to imagine the transpalatal width would be the same for a 6′4" NFL linebacker as for a 5′ ballerina.
Tamburrino and colleagues proposed using each patient as an individual standard, demonstrating three different methods of measuring the relative width of the upper and lower jaws independent of the teeth.47 Each method found that the maxilla was 3-5mm wider than the mandible. The easiest way to determine this measurement is from a CBCT, but there are other methods described in the article that don’t require one. In any case, it makes sense to use the patient as a standard to avoid a population bias and to determine whether the problem is a narrow maxilla (indicating sutural expansion) or dental compensation, as in the inferior convergent group (indicating arch expansion).
Sutural Expansion vs. Arch Expansion
With rare exceptions, fixed expanders will both expand the maxilla and tip teeth. The relative amounts depend on where the force is directed, whether the appliance is fixed or removable, and the age of the patient. In general, the higher the expansion screw relative to the centers of rotation (position 1 or 2), the more force is directed toward maxillary sutural expansion (Fig. 5). The more inferior the expansion screw (positions 4, 5, or 6), the more the forces will tip the teeth. Also, the lower the position of the expansion screw, the more “tongue space” it occupies, thus exacerbating OSA. Fixed expanders direct more force toward sutural expansion than removable appliances. Younger patients also tend toward more sutural expansion than more mature patients, whose sutures fuse at about age 14-15 in females and 15-16 in males.48
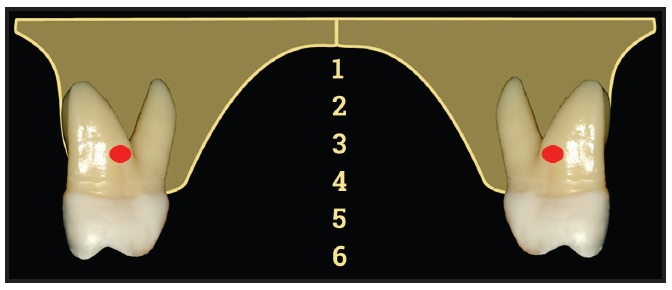
Fig. 5 Cross-section of upper arch indicating centers of rotation of maxillary molars (courtesy of Dr. Ryan Tamburrino).
All studies with before-and-after PSGs have involved fixed expanders, turned at least once a day, with subjects ranging in age from 7 to 14. To make sense of the claims, however, it is useful to differentiate the type of expansion in terms of how the appliance is attached (fixed or removable), how it is turned (rapidly or slowly), the position of the expansion screw relative to the center of rotation, the age of the patient, and the amount of facial growth remaining. It’s a daunting task to sort through all the variables.
So does expansion really affect OSA? The answer is maybe and perhaps minimally, depending on what is meant by “expansion.” The true test for sleep apnea is the overnight sleep study. There are a number of studies49-52 showing a reduction in AHI after rapid maxillary expansion (RME), with improvements persisting at least 24 months past RME treatment.51 There are also numerous studies showing a reduction in OSA symptoms,50,53 nasal airflow resistance,54 and bedwetting53-57 after RME, which has numerous other positive effects on children’s health.58
There are also studies that have compared RME to slow maxillary expansion (SME) and suggested that the two approaches affect the maxillary structures similarly. Whether this translates to an equivalent improvement in OSA has not been demonstrated.59 Turning the screw twice a day for six days, followed by three turns a week for three to four months, caused intraoral effects similar to those of RME—namely, anterior displacement of the maxilla.57,60 Martina and colleagues (using CBCT) noted similar effects from RME and SME on maxillary structures in 10-year-olds.61 Wong found the same pattern of results in 100 children with a mean age of 7.7.62 I have not seen any studies of SME using before-and-after PSGs. All the effects noted were anatomical; because of the age differences of the subjects, the variance in what constituted slow expansion, and the different methods of measurement, it is difficult to compare SME to RME.
More recently, there appears to be a movement toward using archwires and/or removable appliances to expand the maxilla—or even myofunctional therapy to position the tongue palatally and thereby expand the maxilla—under the erroneous belief that expansion is positive and extractions are negative. My practice has case reports demonstrating a dramatic improvement in AHI after only alignment and development of “collapsed” archforms, with an AHI of 52 before treatment and 6 after treatment.34
There is also evidence that sutural expansion increases nasal airflow and reduces nasal resistance to some extent.63-65 This seems to be the biggest differentiator between sutural expansion and arch expansion. If we not only make the tongue space bigger, but also increase nasal airflow, we get double the effect. Simply normalizing the size of the jaw doesn’t always result in proper tongue posture on swallowing. While myofunctional therapy has limited value as a stand-alone solution for OSA,66 it is a wonderful adjunct for retraining the tongue after expansion.
One of the newest additions to the expansion arsenal is the temporary anchorage device (TAD)-supported expander.67,68 Such devices are often positioned within 1mm of the palate; rather than pushing on teeth for anchorage, they attach directly to the palatal bone. As a result, all the expansion force is directed toward sutural expansion and virtually none toward the dentition, providing a dramatic enlargement of the nasal airway in addition to the tongue space. In addition, TAD-supported expanders open the door to nonsurgical sutural expansion in young adults, with a concomitant improvement in OSA measurements and symptoms.
Finally, a word about the multitude of new so-called expansion devices being touted as the latest, or biomimetic, solutions for sleep apnea. Nearly all of these are removable, with either a built-in expansion screw or finger springs to move teeth and “expand” the jaw. I would suggest that these are not really new—they are a step back to a time before modern techniques were developed. There are no studies based on PSG data to confirm the efficacy of these devices. More important, because they primarily tip teeth, they are unlikely to improve nasal airflow; they might also push teeth out of the bony housing and lead to dehiscences and recession, and they may introduce balancing interferences due to tipping. Most critical, while getting some improvement in airway, we may miss the opportunity to make as much improvement as possible. Any one-size-fits-all solution, regardless of how it is marketed, should be used with caution. The process should start with diagnosis, followed by the best treatment for each individual patient.
Tongue Ties
Ankylogossia has been related both to OSA14 and to the development of orthodontic issues.69,70 It is relatively straightforward to correct this problem in an orthodontic office using a soft-tissue laser.71 Once the lingual attachment evaluation is a routine part of the orthodontic examination, this can become a helpful way to improve patients’ lives.
Extractions
Since the subject of expansion vs. extractions is a highly debated issue, a word about the studies we have regarding extractions and OSA. Stefanovic and colleagues compared 31 approximately 13-year-old orthodontic patients, including a group who had four premolars extracted and a group of nonextraction controls matched for age, sex, and pharyngeal airway size. Neither group showed any airway issues before or after treatment; both groups showed significantly enlarged nasopharyngeal and oropharyngeal volumes as well as increased MCA. The authors’ conclusion: “This study suggests that either an extraction or non-extraction choice for orthodontic treatment would not differently affect the pharyngeal airway.”72 Since we know that the airway is growing at this age,45 it is not particularly surprising to find that MCA increased. When one notes that the MCAs ranged from 55.05mm2 to 378.32mm2 at the beginning of treatment, with a similar discrepancy at the end of treatment, one has to wonder whether there was a functional difference between the high and low MCAs.
Pliska and colleagues retrospectively studied 26 adults who had at least two premolars extracted for orthodontic care, comparing their before-and-after CBCT airway measurements to those of 48 nonextraction controls.73 They found no statistically significant differences between the average before-and-after airway volumes or MCAs in the two groups, concluding: “These results suggest that dental extractions in conjunction with orthodontic treatment have a negligible effect on the upper airway in adults.” Note, however, that the mean total airway MCA at T0 was 206.6mm2, with a standard deviation of 98.4mm2; at the end of treatment, mean total airway MCA was 160.6mm2, with a standard deviation of 92.4mm2. The treatment change in total airway MCA for the extraction group was –33.1mm2, with a standard deviation of 53.4mm2. I suspect the subject with an MCA of 68.2mm2 would have a very different breathing ability from the one with an MCA of 253mm2 (±1 standard deviation).
Larson and colleagues retrospectively compared a sample of 2,792 adults, 40-70 years old, who had at least one premolar extracted in each quadrant, to a control group of the same number, matched for body mass index.74 There was no appreciable difference in the percentage of people who had OSA between the extraction group (10.71%) and the control group (9.56%).
What these studies have in common is that they compared population averages with relatively high standard deviations. They were not able to control all the confounding factors, such as before-and-after weight gain, amount of dental crowding, severity of OSA, or number of subjects never tested for OSA. Nonetheless, an AAO white paper concluded that no orthodontic treatments, including extractions, have been shown to cause or increase the likelihood of OSA.75
Orthognathic Surgery
The most predictable orthodontic-related solution for sleep apnea, at 93-100% effectiveness, is a combination of orthodontics with double-jaw surgery.32,76,77 In most such cases, both the maxilla and the mandible are brought forward, but care must be taken to preserve the esthetics of the face and nose. In many patients, normalizing the palatal plane and then bringing the mandible forward in a counterclockwise movement will maximize the advancement without detracting from esthetics.
In the past, we would align the teeth first and plan the surgery for 12-18 months into orthodontic treatment. Today, with our enhanced technologies, many practitioners are choosing a “surgery first” approach (described elsewhere in this issue of JCO). Essentially, the orthodontist places brackets and a passive wire with hooks, then the surgeon normalizes jaw positions and corrects the apnea. After surgery, the orthodontist finishes alignment, but accelerates wire changes to take advantage of the regional acceleratory phenomenon.
Conclusion
OSA and SDB are prevalent in our society, and the majority of cases are undiagnosed and untreated. Those suffering from OSA/SDB will experience not only long-term negative consequences, but also a degradation in their day-to-day quality of life. The Wisconsin Cohort Study, analyzing the life expectancy of 1,522 subjects, found that subjects with severe OSA were 35% less likely to be alive 18 years later, compared to those with normal AHI values.78
The most common solutions for OSA either require patients to wear appliances for the rest of their lives or are generally ineffective. While the diagnosis is not ours to make, we orthodontists have an opportunity to improve our patients’ health, in addition to aligning their teeth, by screening all patients and family members for airway issues. As orthodontists, we can alter the anatomy in a positive way that can reduce the severity of OSA/SDB symptoms over the long term. In most situations, however, it will require more than one treatment to solve OSA, making it crucial for every orthodontist to develop a network of other professionals.
FOOTNOTES
- *Reprinted from the Journal of Oral and Maxillofacial Surgery, volume 70, Schendel, S.A.; Jacobson, R.; and Khalessi, S.: Airway growth and development: A computerized 3-dimensional analysis, 2174-2183, © 2012, with permission from Elsevier. https://www.sciencedirect.com/journal/journal-of-oral-and-maxillofacial-surgery
- **Inspire Medical Systems, Inc., Minneapolis, MN; www.inspiresleep.com.
- ***Space Maintainers Laboratories, Chatsworth, CA; www.smlglobal.com.
- †Trademark of Henry Schein Orthodontics, Carlsbad, CA; www.henryscheinortho.com.
- ‡Modified from the American Journal of Orthodontics and Dentofacial Orthopedics, volume 142, Miner, R.M; Al Qabandi, S.; Rigali, P.H.; and Will, L.A.: Cone-beam computed tomography transverse analysis, Part 1: Normative data, 300-307, © 2012, with permission from Elsevier. https://www.sciencedirect.com/journal/american-journal-of-orthodontics-and-dentofacial-orthopedics
REFERENCES
- 1. Lugaresi, E.; Cirignotta, F.; Coccagna, G.; and Piana, C.: Some epidemiological data on snoring and cardiocirculatory disturbances, Sleep 3:221-224, 1980.
- 2. Partinen, M. and Palomaki, H.: Snoring and cerebral infarction, Lancet 326:1325-1326, 1985.
- 3. Koskenvuo, M.; Kaprio, J.; Partinen, M.; Langinvainio, H.; Sarna, S.; and Heikkilä, K.: Snoring as a risk factor for hypertension and angina pectoris, Lancet 325:893-896, 1985.
- 4. Norton, P.G. and Dunn, E.V.: Snoring as a risk factor for disease: An epidemiological survey, Br. Med. J. 291:630-632, 1985.
- 5. Chervin, R.D.; Dillon, J.E.; Bassetti, C.; Ganoczy, D.A.; and Pituch, K.J.: Symptoms of sleep disorders, inattention and hyperactivity in children, Sleep 20:1185-1192, 1997.
- 6. Halbower, A.C.; Degaonkar, M.; Barker, P.B.; Earley, C.J.; Marcus, C.L.; Smith, P.L.; Prahme, M.C.; and Mahone, E.M.: Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury, PLoS Med. 3:e301, 2006.
- 7. Chan, M.D.; Edman, M.D.; and Koltai, M.D.: Obstructive sleep apnea in children, Am. Fam. Phys. 69:1147-1154, 2004.
- 8. Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N.; Lehmann, C.; and Spruyt, K.: Diagnosis and management of childhood obstructive sleep apnea syndrome, Pediat. 130:576-584, 2012.
- 9. Ricketts, R.M.: Respiratory obstruction syndrome, Am. J. Orthod. 54:495-507, 1968.
- 10. Hawke, M.; Bingham, B.; Stammberger, H.; and Benjamin, B., Diagnostic Handbook of Otorhinolaryngology, Informa Healthcare, London, 1997.
- 11. Hueston, C.K.; Mabry, R.L.; and Mabry, C.S., Allergy in ENT Practice: The Basic Guide, Thieme, New York, 2011.
- 12. Lee, K.J., Essential Otolaryngology: Head and Neck Surgery, McGraw-Hill Professional, New York, 2003.
- 13. Torborg, L.: Mayo Clinic Q and A: Neck size one risk factor for obstructive sleep apnea, Mayo Clinic News Network, June 20, 2015, https://newsnetwork.mayoclinic.org/discussion/mayo-clinic-q-and-a-neck-size-one-risk-factor-for-obstructive-sleep-apnea, accessed Dec. 16, 2021.
- 14. Yoon, A.J.; Zaghi, S.; Ha, S.; Law, C.S.; Guilleminault, C.; and Liu, S.Y.: Ankyloglossia as a risk factor for maxillary hypoplasia and soft palate elongation: A functional-morphological study, Orthod. Craniofac. Res. 20:237-244, 2017.
- 15. Obstructive sleep apnea in children, Stanford Children’s Health, https://www.stanfordchildrens.org/en/topic/default?id=obstructive-sleep-apnea-90-P02026, accessed Dec. 16, 2021.
- 16. Brodsky, L.: Modern assessment of tonsils and adenoids, Pediat. Clin. N. Am. 36:1551-1569, 1989.
- 17. Shaker, A. and Magdy, M.: Frequency of obstructive sleep apnea (OSA) in patients with gastroesophageal reflux disease (GERD) and the effect of nasal continuous positive airway pressure, Egypt. J. Chest Dis. Tuberc. 65:797-803, 2016.
- 18. Bellerive, A.; Montpetit, A.; El-Khatib, H.; Carra, M.C.; Remise, C.; Desplats, E.; and Huynh, N.: The effect of rapid palatal expansion on sleep bruxism in children, Sleep Breath. 19:1265-1271, 2015.
- 19. Manfredini, D.; Colonna, A.; Bracci, A.; and Lobbezoo, F.: Bruxism: A summary of current knowledge on aetiology, assessment and management, Oral Surg. 13:358-370, 2019.
- 20. Battagel, J.M.; Johal, A.; Smith, A.M.; and Kotecha, B.: Postural variation in oropharyngeal dimensions in subjects with sleep disordered breathing: A cephalometric study, Eur. J. Orthod. 24:263-276, 2002.
- 21. Smith, J.M.: The normal adult airway in 3-dimensions: A cone-beam computed tomography evaluation establishing normative values, master’s thesis, University of Michigan, Ann Arbor, MI, 2009.
- 22. Avrahami, E. and Englender, M.: Relation between CT axial cross-sectional area of the oropharynx and obstructive sleep apnea syndrome in adults, AJNR Am. J. Neuroradiol. 16:135-140, 1995.
- 23. Schendel, S.A.; Jacobson, R.; and Khalessi, S.: Airway growth and development: A computerized 3-dimensional analysis, J. Oral Maxillofac. Surg. 70:2174-2183, 2012.
- 24. Iwasaki, T.; Saitoh, I.; Takemoto, Y.; Inada, E.; Kakuno, E.; Kanomi, R.; Hayasaki, H.; and Yamasaki, Y.: Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study, Am. J. Orthod. 143:235-245, 2013.
- 25. Raphael, B.D.; Cruz, M.A.; Roblee, R.D.; and Crean-Binion, E.: Asking the right questions about airway in orthodontics, Orthod. Pract. U.S. 12:10-14, 2021.
- 26. Imayama, I. and Prasad, B.: Role of leptin in obstructive sleep apnea, Ann. Am. Thorac. Soc. 14:1607-1621, 2017.
- 27. Harsch, I.A.; Konturek, P.C.; Koebnick, C.; Kuehnlein, P.P.; Fuchs, F.S.; Schahin, S.P.; Wiest, G.H.; Hahn, E.G.; Lohmann, T.; and Ficker, J.H.: Leptin and ghrelin levels in patients with obstructive sleep apnoea: Effect of CPAP treatment, Eur. Respir. J. 22:251-257, 2003.
- 28. Gagnadoux, F.; Rakotonanahary, D.; Martins de Araujo, M.T.; Barros-Vieira, S.; and Fleury, B.: Long-term efficacy of fixed CPAP recommended by Autoset for OSAS, Sleep 22:1095-1099, 1999.
- 29. Lin, H.S.; Prasad, A.S.; and Pan, C.J.G.: Factors associated with noncompliance to treatment with positive airway pressure, Arch. Otolaryngol. Head Neck Surg. 133:69-72, 2007.
- 30. Steiropoulos, P.; Kotsianidis, I.; Nena, E.; Tsara, V.; Gounari, E.; Hatzizisi, O.; Kyriazis, G.; Christaki, P.; Froudarakis, M.; and Bouros, D.: Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome, Sleep 32:537-543, 2009.
- 31. Roberts, S.D.; Kapadia, H.; Greenlee, G.; and Chen, M.L.: Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions, J. Clin. Sleep Med. 12:469-475, 2016.
- 32. Sher, A.E.; Schechtman, K.B.; and Piccirillo, J.F.: The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome, Sleep 19:156-177, 1996.
- 33. Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; and Cistulli, P.A.: Oral appliance treatment for obstructive sleep apnea: An update, J. Clin. Sleep Med. 10:215-227, 2014.
- 34. Chmura, L.: Sleep apnea: What every clinician (and patient) should know, in Obstructive Sleep Apnea: A Patient’s Perspective, ed. J.A. McNamara Jr. and A.V. Shelgikar, Craniofacial Growth Series, vol. 54, University of Michigan, Ann Arbor, MI, 2018, pp. 175-211.
- 35. Muñoz, I.C.L. and Orta, P.B.: Comparison of cephalometric patterns in mouth breathing and nose breathing children, Int. J. Pediat. Otorhinolaryngol. 78:1167-1172, 2014.
- 36. Alves, M.T.; Baratieri, C.; Nojima, L.I.; Nojima, M.C.G.; and Ruellas, A.C.O.: Three-dimensional assessment of pharyngeal airway in nasal- and mouth-breathing children, Int. J. Pediat. Otorhinolaryngol. 75:1195-1199, 2011.
- 37. Hakan, E.l. and Palomo, J.M.: Three-dimensional evaluation of upper airway following rapid maxillary expansion: A CBCT study, Angle Orthod. 84:265-273, 2013.
- 38. Creekmore, T.D. and Radney, L.J.: Frankel appliance therapy: Orthopedic or orthodontic? Am. J. Orthod. 83:89-108, 1983.
- 39. McNamara, J.A. Jr.: Early intervention in the transverse dimension: Is it worth the effort? Am. J. Orthod. 121:572-574, 2002.
- 40. McNamara, J.A. Jr.; Brudon, W.L.; and Kokich, V.G.: Orthodontics and Dentofacial Orthopedics, Needham Press, Ann Arbor, MI, 2001, p. 57.
- 41. Haas, A.J.: Headgear therapy: The most efficient way to distalize molars, Semin. Orthod. 6:79-90, 2000.
- 42. Haas, A.J.: Long-term posttreatment evaluation of rapid palatal expansion, Angle Orthod. 50:189-217, 1980.
- 43. Bellerive, A.; Montpetit, A.; El-Khatib, H.; Carra, M.C.; Remise, C.; Desplats, E.; and Huynh, N.: The effect of rapid palatal expansion on sleep bruxism in children, Sleep Breath. 19:1265-1271, 2015.
- 44. Trevizan, M.; Nelson, P.F.; Franzolin, S.O.B.; and Consolaro, A.: Premaxilla: Up to which age it remains separated from the maxilla by a suture, how often it occurs in children and adults, and possible clinical and therapeutic implications: Study of 1,138 human skulls, Dent. Press J. Orthod. 23:16-29, 2018.
- 45. Miner, R.M.; Al Qabandi, S.; Rigali, P.H.; and Will, L.A.: Cone-beam computed tomography transverse analysis, Part I: Normative data, Am. J. Orthod. 142:300-307, 2012.
- 46. McNamara, J.A. Jr.: Maxillary transverse deficiency, Am. J. Orthod. 117:567-570, 2000.
- 47. Tamburrino, R.K.; Boucher, N.S.; Vanarsdall, R.L.; and Secchi, A.: The transverse dimension: Diagnosis and relevance to functional occlusion, Roth Williams Int. Soc. Orthod. J. 2:11-20, 2010.
- 48. Haas, A.J.: Long-term posttreatment evaluation of rapid palatal expansion, Angle Orthod. 50:189-217, 1980.
- 49. Pirelli, P.; Saponara, M.; and Guilleminault, C.: Rapid maxillary expansion in children with obstructive sleep apnea syndrome, Sleep 27:761-766, 2004.
- 50. Villa, M.P.; Malagola, C.; Pagani, J.; Montesano, M.; Rizzoli, A.; Guilleminault, C.; and Ronchetti, R.: Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up, Sleep Med. 8:128-134, 2007.
- 51. Villa, M.P.; Rizzoli, A.; Miano, S.; and Malagola, C.: Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up, Sleep Breath. 15:179-184, 2011.
- 52. Cistulli, P.A.; Palmisano, R.G.; and Poole, M.D.: Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion, Sleep 21:831-835, 1998.
- 53. Miano, S.; Rizzoli, A.; Evangelisti, M.; Bruni, O.; Ferri, R.; Pagani, J.; and Villa, M.P.: NREM sleep instability changes following rapid maxillary expansion in children with obstructive apnea sleep syndrome, Sleep Med. 10:471-478, 2009.
- 54. Bazargani, F.; Jönson-Ring, I.; and Nevéus, T.: Rapid maxillary expansion in therapy-resistant enuretic children: An orthodontic perspective, Angle Orthod. 86:481-486, 2016.
- 55. Timms, D.J.: Rapid maxillary expansion in the treatment of nocturnal enuresis, Angle Orthod. 60:229-233, 1990.
- 56. Usumez, S.; Işeri, H.; Orhan, M.; and Basciftci, F.A.: Effect of rapid maxillary expansion on nocturnal enuresis, Angle Orthod. 73:532-538, 2003.
- 57. Kurol, J.; Modin, H.; and Bjerkhoel, A.: Orthodontic maxillary expansion and its effect on nocturnal enuresis, Angle Orthod. 68:225-232, 1998.
- 58. Eichenberger, M. and Baumgartner, S.: The impact of rapid palatal expansion on children’s general health: A literature review, Eur. J. Paediat. Dent. 15:67-71, 2014.
- 59. Kilic, N. and Oktay, H.: Effects of rapid-slow maxillary expansion on the dentofacial structures, Aust. Orthod. J. 26:178-183, 2010.
- 60. Corbridge, J.K.; Campbell, P.M.; Taylor, R.; Ceen, R.F.; and Buschang, P.H.: Transverse dentoalveolar changes after slow maxillary expansion, Am. J. Orthod. 140:317-325, 2011.
- 61. Martina, R.; Cioffi, I.; Farella, M.; Leone, P.; Manzo, P.; Matarese, G.; Portelli, M.; Nucera, R.; and Cordasco, G.: Transverse changes determined by rapid and slow maxillary expansion: A low-dose CT-based randomized controlled trial, Orthod. Craniofac. Res. 15:159-168, 2012.
- 62. Wong, C.A.; Sinclair, P.M.; Keim, R.G.; and Kennedy, D.B.: Arch dimension changes from successful slow maxillary expansion of unilateral posterior crossbite, Angle Orthod. 81:616-623, 2011.
- 63. Bazargani, F.; Magnuson, A.; and Ludwig, B.: Effects on nasal airflow and resistance using two different RME appliances: A randomized controlled trial, Eur. J. Orthod. 40:281-284, 2018.
- 64. Iwasaki, T.; Takemoto, Y.; Inada, E.; Sato, H.; Suga, H.; Saitoh, I.; Kakuno, E.; Kanomi, R.; and Yamasaki, Y.: The effect of rapid maxillary expansion on pharyngeal airway pressure during inspiration evaluated using computational fluid dynamics, Int. J. Pediat. Otorhinolaryngol. 78:1258-1264, 2014.
- 65. Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papageorgiou, S.N.; and Papadopoulou, A.K.: Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis, Eur. J. Orthod. 39:463-473, 2017.
- 66. Camacho, M.; Certal, V.; Abdullatif, J.; Zaghi, S.; Ruoff, C.M.; Capasso, R.; and Kushida, C.A.: Myofunctional therapy to treat obstructive sleep apnea: A systematic review and meta-analysis, Sleep 38:669-675, 2015.
- 67. Evans, M.: Three-dimensional control with TAD-tissue supported rapid palatal expander: An overview of clinical applications and biological advantages, Orthod. Clin. Rev. 22-31, 2013, https://www.rmortho.com/wp-content/uploads/2013/08/Clinical_Final_LR.pdf, accessed Dec. 16, 2021.
- 68. Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; and Moon, W.: Non-surgical treatment of transverse deficiency in adults using microimplant-assisted rapid palatal expansion (MARPE), Dent. Press J. Orthod. 22:110-125, 2017.
- 69. Lalakea, M.L. and Messner, A.H.: Ankyloglossia: Does it matter? Pediat. Clin. N. Am. 50:381-397, 2003.
- 70. Defabianis, P.: Ankyloglossia and its influence on maxillary and mandibular development (a seven year follow-up case report), Funct. Orthod. 17:25-33, 2000.
- 71. Chmura, L.: “Other procedures” for which your soft tissue laser is useful, Orthotown, June 2010, https://www.orthotown.com/magazine/article/2771/ldquoother-proceduresrdquo-for-which-your-soft-tissue-laser-is-useful, accessed Dec. 16, 2021.
- 72. Stefanovic, N.; El, H.; Chenin, D.L.; Glisic, B.; and Palomo, J.M.: Three-dimensional pharyngeal airway changes in orthodontic patients treated with and without extractions, Orthod. Craniofac. Res. 16:87-96, 2013.
- 73. Pliska, B.T.; Tam, I.T.; Lowe, A.A.; Madson, A.M.; and Almeida, F.R.: Effect of orthodontic treatment on the upper airway volume in adults, Am. J. Orthod. 150:937-944, 2016.
- 74. Larsen, A.J.; Rindal, D.B.; Hatch, J.P.; Kane, S.; Asche, S.E.; Carvalho, C.; and Rugh, J.: Evidence supports no relationship between obstructive sleep apnea and premolar extraction: An electronic health records review, J. Clin. Sleep Med. 11:1443-1448, 2015.
- 75. American Association of Orthodontists: White paper: Obstructive sleep apnea and orthodontics, amended Mar. 15, 2019, www2.aaoinfo.org/wp-content/uploads/2019/03/sleep-apnea-white-paper-amended-March-2019.pdf, accessed Feb. 9, 2022.
- 76. Holty, J.E.C. and Guilleminault, C.: Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis, Sleep Med. Rev. 14:287-297, 2010.
- 77. AlSaty, G.; Xiang, J.; Burns, M.; Eliliwi, M.; Palomo, J.M.; Martin, C.; Weaver, B.; and Ngan, P.: Follow-up observation of patients with obstructive sleep apnea treated by maxillomandibular advancement, Am. J. Orthod. 158: 527-534, 2020.
- 78. Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; and Hla, K.M.: Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort, Sleep 31:1071-1078, 2008.



COMMENTS
.