Tooth Whitening in Association with Clear Aligner Treatment
The ever-increasing patient demand for “invisible” orthodontic appliances has led to major growth in the clear aligner industry.1 Not only do aligners have a minimal esthetic impact, but they are also an efficacious means of moving teeth into the positions prescribed during setup.2-4
The first worldwide commercial aligner system was made by Align Technology using a single-layer rigid polyurethane. Align’s current material is SmartTrack,* a thermoplastic polyurethane with an integrated elastomer specifically engineered for the Invisalign* system.5
The recently introduced F22** aligner, designed by researchers at the University of Ferrara, Italy, is made of a single layer of .75mm-thick, chemically inert polyurethane that has several beneficial properties. First, the flexible material maintains a constant and light stress relaxation over time,6 despite the significant stress decay that all aligners experience during a 24-hour period. In addition, because of the F22’s straight-line gingival margin along the gingival zenith, the aligner has demonstrated stronger retention forces (25N).7 Esthetically, the F22 is highly transparent due to an absence of structural defects, allowing as much as 20% more light to pass through than with other aligners.8
Similar articles from the archive:
- THE READERS' CORNER Tooth-Whitening April 2017
- THE READERS' CORNER Tooth-Whitening and Incisor Positioning October 2003
- Bleaching Teeth During Supervised Retention June 1999
Along with dental alignment, tooth shade is a major factor in the perception of smile esthetics.9 Whitening is being requested more frequently, especially by orthodontic patients10; in one study, 88% of orthodontic patients asked for whitening during or after their treatment.11 Krug and Green found a significantly higher 90% satisfaction rate among patients when whitening was included with orthodontic treatment, compared with orthodontic treatment alone.12
This trend has prompted several investigators to assess the efficacy of whitening agents applied during multibracket appliance treatment.13-15 Results have been mixed, however, and no combination of orthodontic treatment and tooth-whitening strategy has proved entirely satisfactory.
Although conventional whitening trays incorporate reservoirs for the whitening agent, the close fit of a clear aligner—while essential for control and predictability during treatment—leaves little room for a whitening gel. We hypothesized that a uniform distribution could be achieved by spraying a liquid whitening agent directly onto the interior surfaces of the aligner trays. The optimal composition and concentration of an agent that could whiten the teeth without causing deterioration in the microstructure or esthetic qualities of the aligner material had yet to be determined. Therefore, the present study was designed to evaluate different at-home whitening protocols during orthodontic clear aligner treatment.
Materials and Methods
The efficacy of the whitening spray formulation was tested on a sample of 38 volunteers who were undergoing orthodontic treatment with F22 aligners at the University of Ferrara Orthodontic Clinic. An adult over age 18 was included in the study if he or she had six vital maxillary anterior teeth, no need for attachments or divots on the labial surfaces of the upper canines or incisors, no direct or indirect reconstruction of the upper canines or incisors, no history of dentin hypersensitivity, and no history of professional bleaching during the previous three years. Before beginning the treatment, each participant signed an informed-consent document describing the assigned whitening treatment and its potential adverse effects.
The participants were randomized as follows:
- Group 1 (eight patients): Application of 3% hydrogen peroxide for nine hours per day on days 7-14 of aligner wear.
- Group 2 (eight patients): Application of 10% carbamide peroxide for nine hours per day on days 7-14 of aligner wear.
- Group 3 (eight patients): Application of 16% carbamide peroxide for nine hours per day on days 7-14 of aligner wear.
- Group 4 (14 patients): Application of 16% carbamide peroxide for nine hours per day on days 1-14 of aligner wear.
According to previous cone-beam computed tomography analyses, the fit of the F22 aligner provides less than 40µm of space between the tray and the anterior tooth surface.16 Realistically, the thickness of the bleaching solution will never exceed this value. To qualitatively analyze the distribution pattern of a liquid within the aligner, we randomly selected four patients. A blue-dyed whitening solution was applied using the same standardized protocol that would be followed during the efficacy portion of the study. The blue liquid was sprayed onto the internal labial surface of the aligner, with one spray at the central incisors and one spray in each of the canine regions. The upper aligner was fitted in the patient’s upper arch as it would normally be worn, and a frontal photograph was taken to enable visual evaluation of the whitening agent’s distribution pattern between the aligner and the upper anterior teeth.
On the day the patient was provided with the appropriate whitening spray (T1), a spectrophotometric analysis was performed using a VITA Easyshade V*** compact, which was chosen for its proven accuracy and reliability.17,18 Three values were read for each of the six maxillary anterior teeth—one each at the incisal, middle, and cervical thirds of the labial surfaces (Table 1).
The 16 shades of the VITA classical A1-D4*** color scale were reorganized from highest (B1) to lowest (C4), as recommended by the ADA,17 and each value was assigned a numerical equivalent (Table 2).
Comparison of the median shades for each site in the upper anterior segment before whitening, using the Kruskal-Wallis H test, found no statistically significant differences among the four patient samples, making it possible to state that all four groups belonged to the same population.
At the same appointment, the patient was instructed to apply the whitening spray as described above after dinner and toothbrushing, at about 10-11 p.m., and to leave the aligner in place for nine consecutive hours, until about 7-8 a.m.
Each patient returned three days later to be checked for side effects. Because no gingival inflammation or changes in dental sensitivity were detected in any patients, all participants continued following the initial instructions. No changes in the bleaching protocol or prescription of desensitizing agents were required during the study. The color analysis was repeated at the two-week follow-up (T2), following the same protocol as before.
A scanning electron microscope† (SEM) was used to conduct a qualitative analysis of the F22 aligners before and after application of the whitening spray. We tested one unused aligner sample, one aligner that had been worn for 14 days before whitening (T1), and one from each of the four whitening groups at T2. A section measuring 5mm × 5mm was taken from the median third of the aligner’s labial surface at the level of the upper right central incisor. Each sample was gilded, and SEM images were taken at magnifications 250×, 1,000×, and 5,000×.
A double-beam spectrophotometer‡ with an accuracy of 2nm was used to conduct a quantitative analysis of the F22 aligners in association with the whitening spray. Nine randomly selected patients each provided the last-used upper aligners from T1 and T2. The spectrophotometer was calibrated to the white-light spectrum; following the protocol of Lombardo and colleagues, we considered only wavelengths in the visible 400-700nm spectrum.8 The lingual area of each aligner was first sectioned from canine to canine to expose only the labial surface to the spectrophotometer light source. Each aligner was then positioned on a 35mm-high support, so that the labial surface of the upper left central incisor region was vertical and in contact with the spectrophotometer’s incident light collection window (Fig. 1). The light transmittance of each sample was recorded automatically. Each sample was analyzed three times, after slight modifications of the aligner inclinations, for a total of 54 transmittance measurements. Readings were analyzed with Spectra Manager II†† software, which plotted a mean curve for each aligner.
For each group, we used the mean as the measure of central tendency and the relative standard deviation as the measure of variability. The analysis of variance F test (p < .05) and Bonferroni post hoc test (p < .05) were used to identify any statistically significant differences among the mean percentage variations in each group. Nonparametric evaluations were chosen because the data did not fit a normal distribution.
A binomial z-test was performed, using the mean values for each test group, to identify the at-home whitening protocols that had resulted in statistically significant improvements in whiteness (p < .05). The percentage variation from T1 was then calculated for each site, with B1 as the upper limit of 100%, to better delineate the degree of whitening observed in each group. The Kolmogorov-Smirnov goodness-of-fit test was used to determine which variations did not display significantly different distributions from normal (p < .05).
Results
In the qualitative analysis of whitening agent distribution within the F22 aligners, the frontal photographs indicated uniform distribution of the blue-dyed whitening sprays across the upper anterior tooth surfaces (Fig. 2).
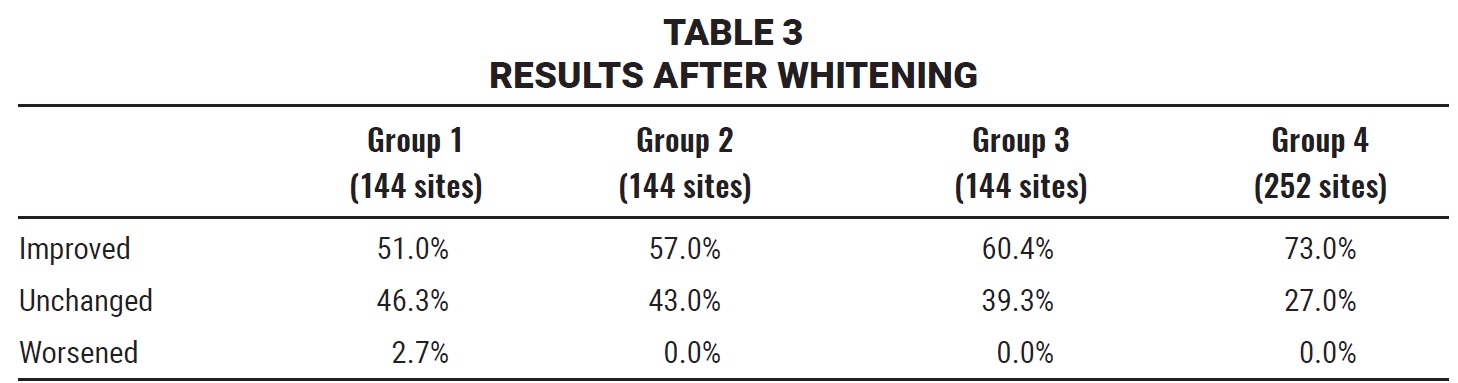
A total of 144 sites for each of the first three groups and 252 for the fourth group were evaluated for the efficacy of whitening sprays by comparing the values obtained at T1 to those at T2 (Table 3).
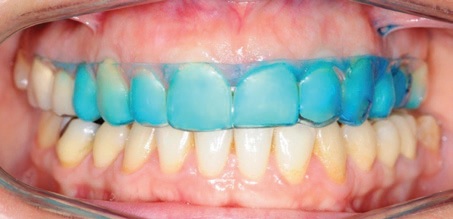
Fig. 2 Uniform distribution of blue-dyed whitening agent within aligner.
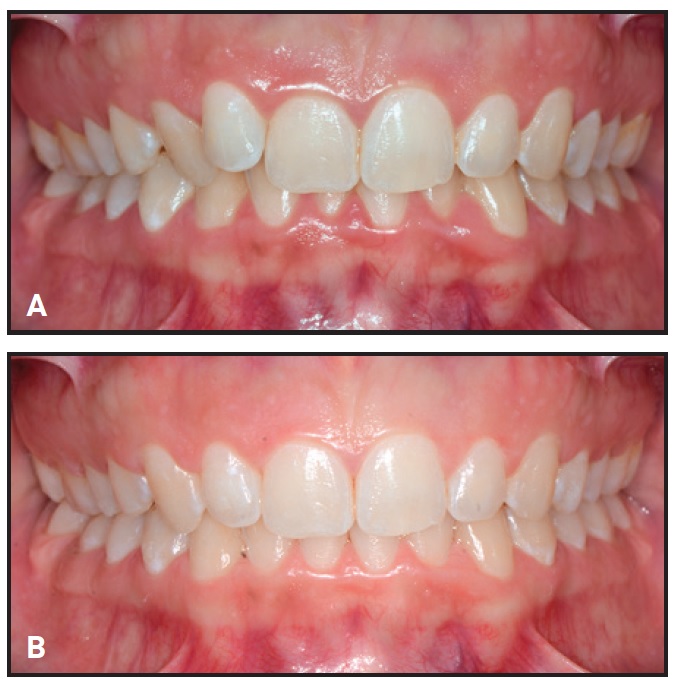
Fig. 3 Representative patient from Group 1 before (A) and after (B) combined clear aligner and whitening treatment.
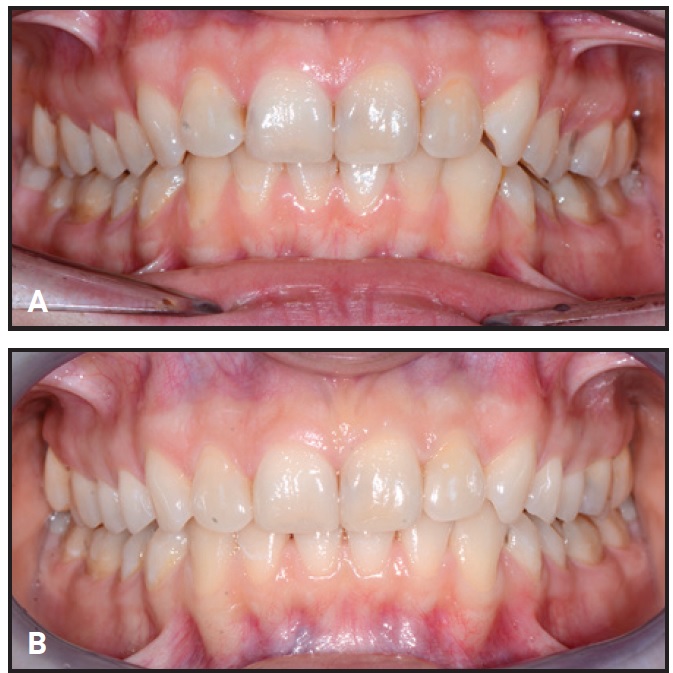
Fig. 4 Representative patient from Group 4 before (A) and after (B) combined clear aligner and whitening treatment.
Only Group 1 included any sites that worsened in color after whitening treatment (2.7%). On the other hand, only Groups 3 (p = .013) and 4 (p < .0001) showed significantly greater numbers of whiter sites relative to unchanged sites. Group 4 patients achieved a significantly greater shade improvement (Fig. 5) than patients in Groups 1 (p = .02), 2 (p = .01), or 3 (p = .01).
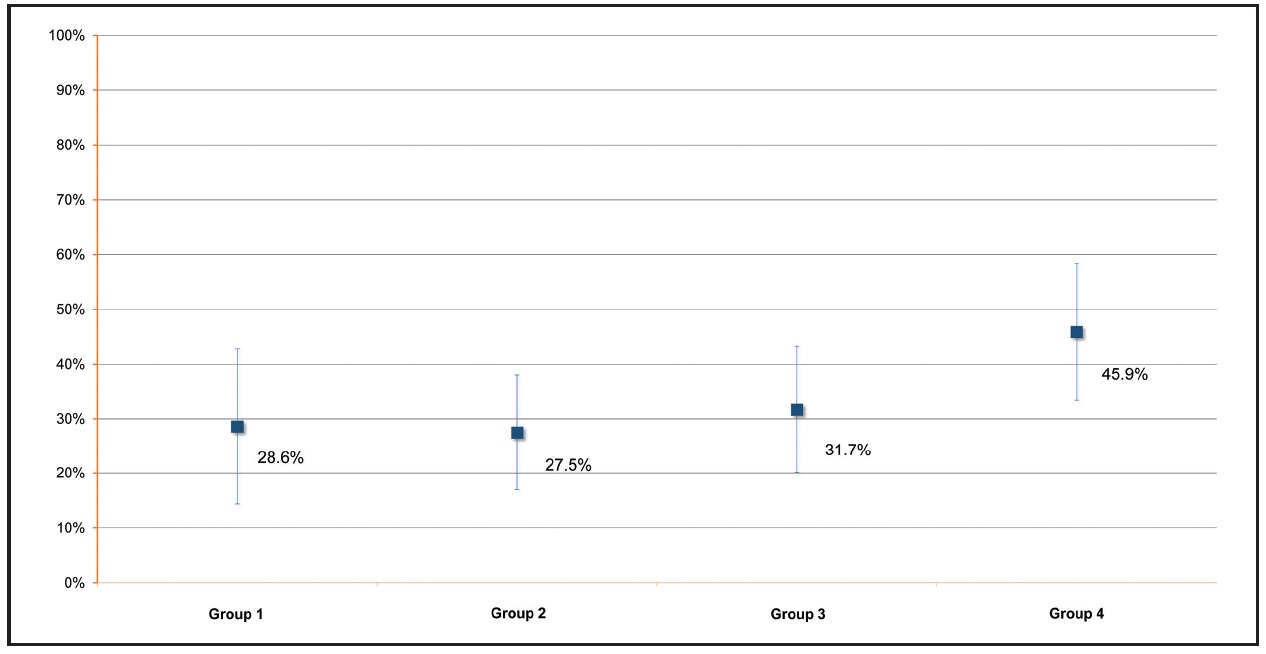
Fig. 5 Mean shade variations after whitening treatment (T2), with VITA classical shade B1*** designated as 100%.
In the SEM analysis of F22 aligners after whitening agent application, the six samples showed no appreciable microstructural differences at magnifications 250×, 1,000×, or 5,000×. In every case, the filamentous appearance typical of the aligner material (Fig. 6) was hidden by a continuous layer of organic material, deposited during normal wear. Crystalline deposits of mineral salts were evident at higher magnifications (Fig. 7).
In the spectrophotometric analysis of nine aligners, the aligners maintained their transparency after 14 days of using either a hydrogen peroxide or carbamide peroxide whitening agent (Table 4). The amount of light passing through the aligner was greater than 80% at T1 and remained high at T2, as demonstrated by the mean transmittance curves for the visible wavelength range of 400-700nm (Fig. 8).
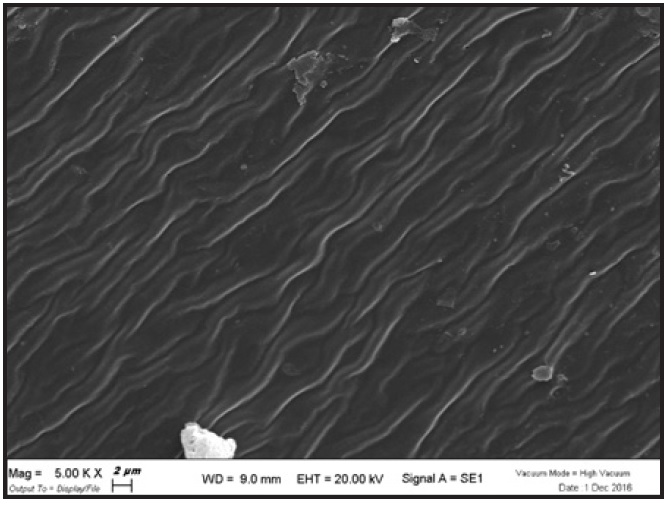
Fig. 6 F22 aligner material before use, as seen under scanning electron microscope† at magnification 5,000×.
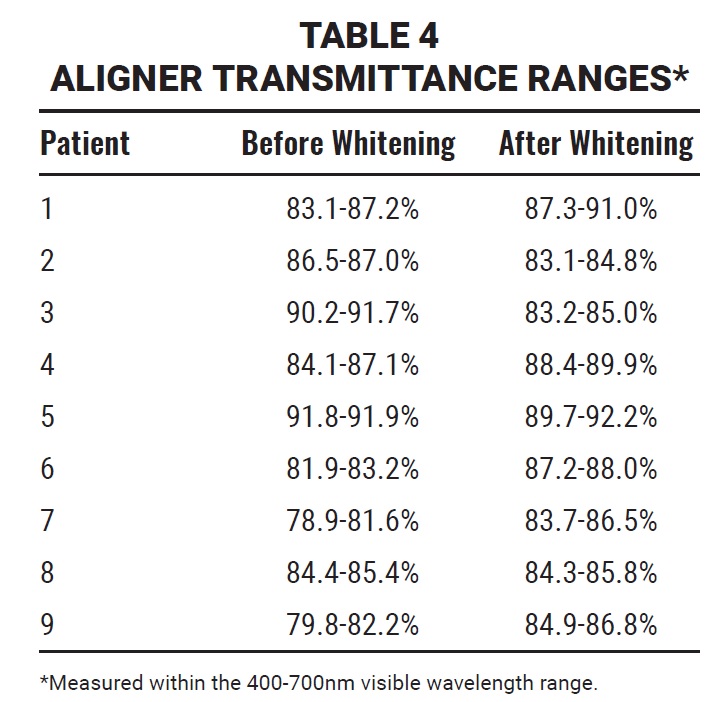
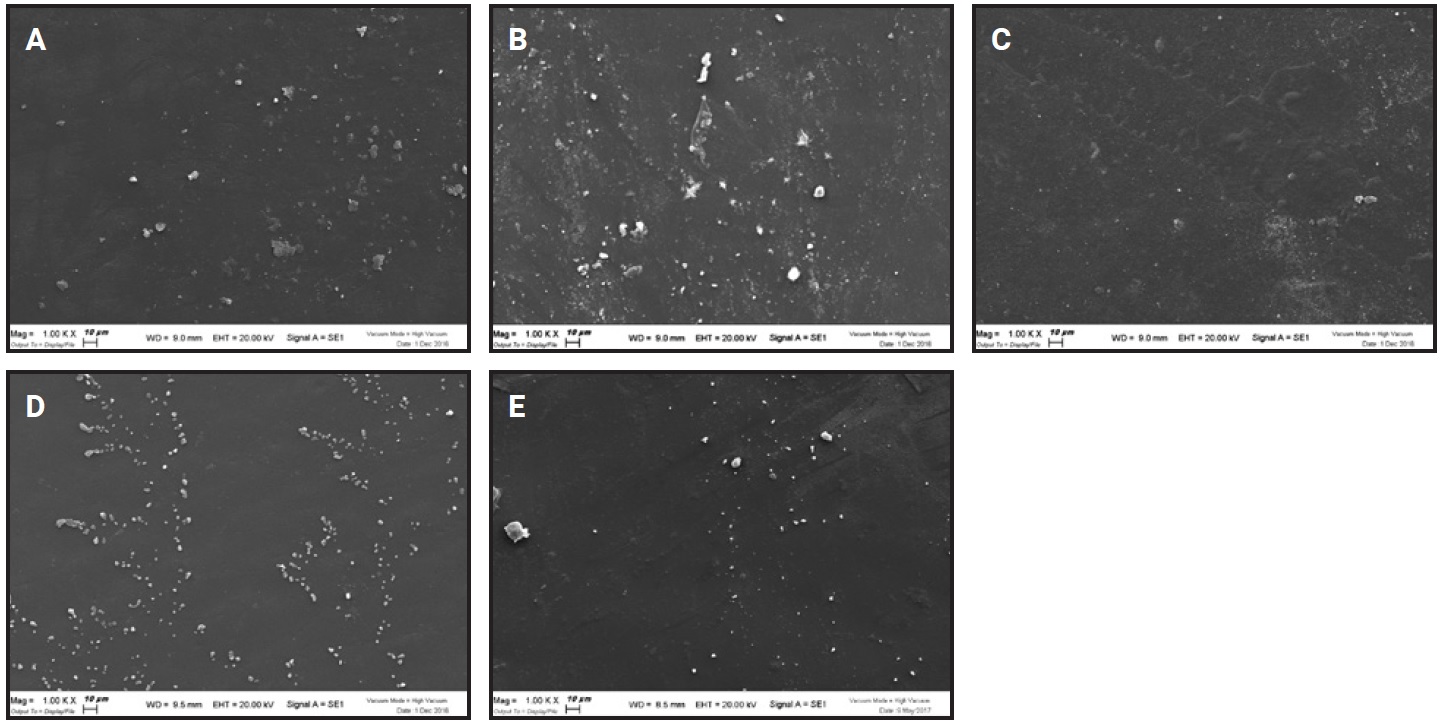
Fig. 7 Aligner material at magnification 1,000× before application of whitening agent (A) and in Groups 1 (B), 2 (C), 3 (D), and 4 (E) after 14 days of wear.
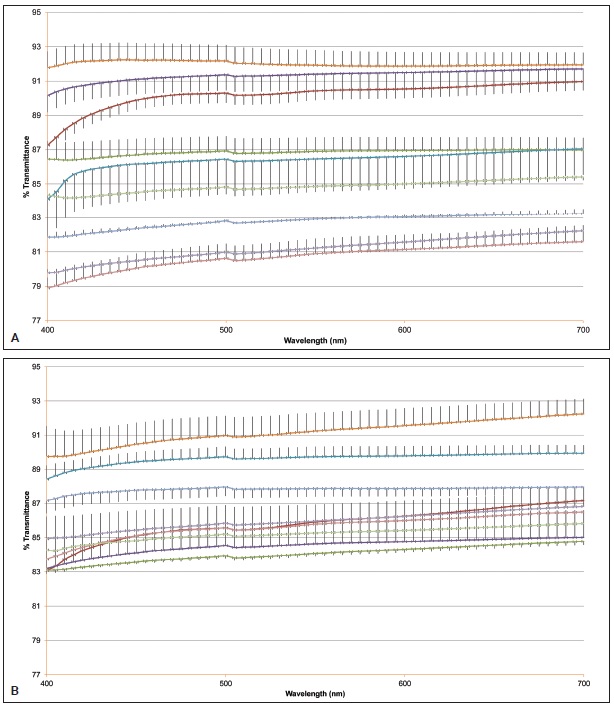
Fig. 8 A. Mean transmittance curves of nine aligners at T1 (wavelength range 400-700nm). B. Mean transmittance curves of nine aligners at T2 (wavelength range 400-700nm).
Discussion
Simultaneous orthodontic and whitening treatment is likely to be well received by patients, since it combines two major factors involved in smile esthetics.10,19 This is particularly important because patient compliance plays a decisive role in the outcome of aligner treatment.
At-home whitening procedures can provide excellent results, often better than with in-office treatment.20 Moreover, whitening agents that can be applied at home are associated with fewer adverse effects, as they generally contain lower concentrations of the active peroxide ingredient.21
After confirming that liquid whitening agents could be evenly distributed within the small space between F22 aligner trays and the teeth, we tested the efficacy of various concentrations of sprayed whitening agents. Our results confirmed other reports that the concentration of whitening agent and the length of time in contact with the teeth are the key factors.22,23 We found that the greatest percentage improvement in whiteness was achieved with the application of 16% carbamide peroxide for nine hours per day over 14 days (Group 4).
SEM analysis indicated that the whitening agents had no effect on the F22 aligner material, because the microfilamentous structure was covered by a layer of organic material. Although some surface irregularities were visible at high magnifications, these appear to have been caused by the heat applied during the gilding process, rather than by chemical degradation. Striations that probably resulted from normal cleaning were also visible.
Following an established protocol,8 we used a high-performance spectrophotometer to compare the light transmittance and absorbance of the aligners before and after whitening. The F22 aligners maintained a high level of transparency—more than 80%—even after 14 days of wear. The stereolithographic manufacturing process has been shown to produce thermoplastic polyurethane aligners with very smooth surfaces6,8; in our study, neither whitening agent (hydrogen peroxide or carbamide peroxide) altered these characteristics.
Our participants, similar to those assessed by Krug and Green,12 reported a high degree of satisfaction and were particularly pleased that the whitening and alignment were performed simultaneously, without additional chairtime. Even though the aligner tray provides no reservoir for the bleaching agent, we did not observe any intolerable side effects from the use of peroxide.
This study presents some limitations. First, it would have benefited from a larger and more homogeneous sample. Another drawback was the lack of a precise protocol for randomization and selection of the sample size. While the number of patients in each group was limited, however, the total number of sites analyzed (144 each in Groups 1, 2, and 3 and 252 in Group 4) was sufficient for a reliable statistical analysis.
A disadvantage of the method itself is that when whitening agents are used in conjunction with aligners, no attachments can be bonded to the labial surfaces of the anterior teeth. After careful analysis by orthodontists, the F22 system was designed to reduce visual impact by using anterior attachments bonded to the lingual surfaces. If the clinician’s planned movements require anterior labial attachments, any tooth whitening should be postponed to the end of treatment by using the final aligner (the one that will serve as a removable post-treatment retainer) as the reservoir for the whitening solution.
Further studies should be conducted to compare the efficacy of common at-home dental whitening protocols in association with various types of clear orthodontic aligners. Collateral effects should also be investigated to determine whether the incidence of dental sensitivity or gingival inflammation is comparable to that of other whitening protocols.24,25
FOOTNOTES
- *Registered trademark of Align Technology, Inc., San Jose, CA; www.aligntech.com.
- **Sweden & Martina SpA, Due Carrare, Italy; www.f22aligner.com.
- ***Registered trademark of Vita North America, Yorba Linda, CA; www.vitanorthamerica.com.
- †Zeiss EVO 40, Carl Zeiss Microscopy, LLC, Thornwood, NY; www.zeiss.com.
- ‡UV-Vis V-630PC, JASCO, Easton, MD; www.jascoinc.com.
REFERENCES
- 1. Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; and Johnston, W.M.: Attractiveness, acceptability, and value of orthodontic appliances, Am. J. Orthod. 135:276, 2009.
- 2. Boyd, R.; Miller, R.J.; and Vlaskalic, V.: The Invisalign system in adult orthodontics: Mild crowding and space closure cases, J. Clin. Orthod. 154:203-212, 2000.
- 3. Kim, T.W. and Echarri, P.: Clear aligner: An efficient, esthetic, and comfortable option for an adult patient, World J. Orthod. 8:13-18, 2007.
- 4. Kohda, N.; Lijima, M.; Muguruma, T.; Brantley, W.A.; Ahluwalia, K.S.; and Mizoguchi, I.: Effects of mechanical properties of thermoplastic materials on the initial force of thermoplastic appliances, Angle Orthod. 83:476-483, 2013.
- 5. Align Technology, Inc.: Align Technology introduces next generation of Invisalign aligner material, www.investor.aligntech.com/news-release, accessed Aug. 4, 2019.
- 6. Lombardo, L.; Martines, E.; Mazzanti, V.; Arreghini, A.; Mollica, F.; and Siciliani, G.: Stress relaxation properties of four orthodontic aligner materials: A 24-hour in vitro study, Angle Orthod. 87:11-18, 2017.
- 7. Cowley, D.P.; Mah, J.; and O’Toole, B.: The effect of gingival-margin design on the retention of thermoformed aligners, J. Clin. Orthod. 46:697-702, 2012.
- 8. Lombardo, L.; Arreghini, A.; Maccarone, R.; Bianchi, A.; Scalia, S.; and Siciliani, G.: Optical properties of orthodontic aligners—Spectrophotometry analysis of three types before and after aging, Prog. Orthod. 16:41, 2015.
- 9. Tin-Oo, M.M.; Saddki, N.; and Hassan, N.: Factors influencing patient satisfaction with dental appearance and treatments they desire to improve aesthetics, BMC Oral Health 11:6, 2011.
- 10. Slack, M.E.; Swift, E.J. Jr.; Rossouw, P.E.; and Phillips, C.: Tooth whitening in the orthodontic practice: A survey of orthodontists, Am. J. Orthod. 143:S64-71, 2013.
- 11. Davis, L.G.; Ashworth, P.D.; and Spriggs, L.S.: Psychological effects of aesthetic dental treatment, J. Dent. 26:547-556, 1998.
- 12. Krug, A.Y. and Green, C.: Changes in patient evaluation of completed orthodontic esthetics after dental bleaching, J. Esth. Dent. 20:313-319, 2008.
- 13. Jadad, E.; Montoya, J.; Arana, G.; Gordillo, L.A.; Palo, R.M.; and Loguercio, A.D.: Spectrophotometric evaluation of color alterations with a new dental bleaching product in patients wearing orthodontic appliances, Am. J. Orthod. 140:e43-47, 2011.
- 14. Lunardi, N.; Correr, A.B.; Rastelli, A.N.; Lima, D.A.; and Consani, R.L.: Spectrophotometric evaluation of dental bleaching under orthodontic bracket in enamel and dentin, J. Clin. Exp. Dent. 6:e321-e326, 2014.
- 15. Gomes, M.N.; Dutra, H.; Morais, A.; Sgura, R.; and Devito-Moreas, A.G.: In-office bleaching during orthodontic treatment, J. Esth. Dent. 29:83-92, 2017.
- 16. Arveda, N.: Aligners fitting and retention analysis, thesis, Postgraduate School of Orthodontics, Ferrara University, Ferrara, Italy, 2014.
- 17. Browning, W.D.: Use of shade guides for color measurement in tooth-bleaching studies, J. Esth. Restor. Dent. 15:512-520, 2003.
- 18. Kim-Pusateri, S.; Brewer, J.D.; Davis, E.L.; and Wee, A.G.: Reliability and accuracy of four dental shade-matching devices, J. Prosth. Dent. 101:193-199, 2009.
- 19. Nedwed, V. and Miethke, R.R.: Motivation, acceptance and problems of Invisalign patients, J. Orofac. Orthop. 66:162-173, 2005.
- 20. Zekonis, R.; Matis, B.A.; Cochran, M.A.; Al Shetri, S.E.; Eckert, G.J.; and Carlson, T.J.: Clinical evaluation of in-office and at-home bleaching treatments, Oper. Dent. 28:114-121, 2003.
- 21. Vaz, M.N.; Lopes, L.G.; Cardoso, P.C.; Souza, J.B.; Batista, A.C.; Costa, N.L.; Torres, E.M.; and Estrela, C.: Inflammatory response of human dental pulp to at-home and in-office tooth bleaching, J. Appl. Oral Sci. 24:509-517, 2016.
- 22. Sulieman, M.; Addy, M.; MacDonald, E.; and Rees, J.S.: The effect of hydrogen peroxide concentration on the outcome of tooth whitening: An in-vitro study, J. Dent. 32:295-299, 2004.
- 23. Leonard, R.H.; Sharma, A.; and Haywood, V.B.: Use of different concentration of carbamide peroxide for bleaching teeth: An in vitro study, Quintess. Int. 29:503-507, 1998.
- 24. Tay, L.Y.; Kose, C.; Herrera, D.R.; Reis, A.; and Loguercio, A.D.: Long-term efficacy of in-office and at-home bleaching: A 2-year double-blind randomized clinical trial, Am. J. Dent. 25:199-204, 2012.
- 25. Ly, Y. and Greenwall, L.: Safety issues of tooth whitening using peroxide-based materials, Br. Dent. J. 215:29-34, 2013.








COMMENTS
.