Bisphosphonates and Orthodontics: Clinical Implications
While adult patients have been welcome in most orthodontic practices, they present a number of challenges, not all of which are related to malocclusions. For example, the oral administration of bisphosphonates is rising dramatically in the form of anti-osteoporosis medications, especially in women. Osteonecrosis of the jaws has been associated with intravenous administration of bisphosphonates, and there are also reports of oral lesions from "chronic" oral bisphosphonate use.1 Although many of these cases were associated with dental extractions or mucosal trauma, some of the lesions seem to have appeared spontaneously.2
This article is a brief review of the pharmacology of bisphosphonates and their potential impact on orthodontic patients.
Bisphosphonates
Bisphosphonates are a synthetic class of pyrophosphate analogues that are powerful inhibitors of bone resorption. They have a high affinity for calcium, and are either maintained in the bone, recirculated, or excreted in the urine.3 Because bisphosphonates are not metabolized, high concentrations will persist in the bone for long periods.
Similar articles from the archive:
All bisphosphonates share a common chemical structure, in which two PO3 (phosphate) groups are covalently bound to carbon atoms (Fig. 1). The long (R2) side chain determines the potency of the drug, while the short (R1) side chain influences the pharmacokinetics.4 Either an aminoterminal group or a cyclic-nitrogen-containing chain on the R2 side will increase the resorptive potential logarithmically (Table 1).
The mechanism of action of bisphosphonate drugs is still under investigation, but some basic pathophysiology is understood. Bisphosphonates attach to bone because of their parachlorophenol moiety's affinity for hydroxyapatite, and are then phagocytized by osteoclasts.4 The ingestion of bisphosphonates by osteoclasts triggers apoptosis (programmed cell death) by competing with adenosine triphosphate or interfering with the HMG-CoA reductase pathway.6 Osteoblast-mediated osteoclastic resorption is also inhibited.3 Recent evidence points to an anti-angiogenic mechanism that may reduce the healing potential of already compromised bone by inhibiting vascularization.7
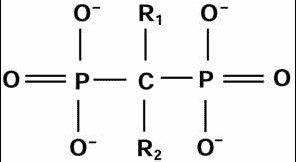
Fig. 1 Basic chemical structure of bisphosphonate.
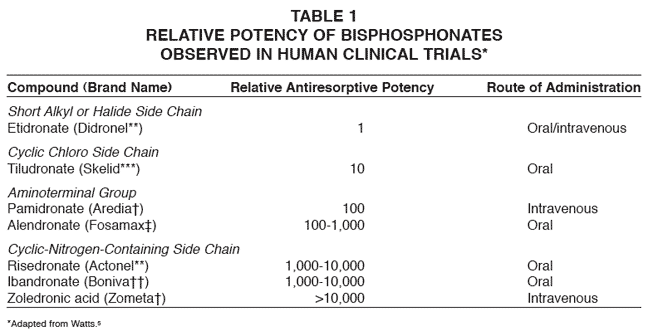
The efficacy of bisphosphonates cannot be denied.8,9 They are commonly used intravenously for treatment of hypercalcemia of malignancy, osteitis deformans ("Paget's disease of bone"), bone metastasis (with or without hypercalcemia), and multiple myeloma, and orally for osteoporosis.10-12 Television ads targeting the demographic group prone to osteoporosis are increasingly common.
Osteonecrosis of the Jaws
In a recent study by Marx and colleagues, 119 well-documented cases of bisphosphonate-induced osteonecrosis of the jaws were reviewed for potential risk factors and etiologies.13 Of course, the underlying malignancies and all their negative sequelae were the greatest risks among these patients. Aggravating factors such as smoking, alcohol use, and ongoing chemotherapy have also been recognized.
Of the 119 cases of osteonecrosis reviewed by Marx and colleagues, 45 cases (37.8%) were related to the removal of a tooth or teeth, 34 (28.6%) to obvious existing periodontal disease, five (11.2%) to periodontal surgery, four (3.4%) to dental implant placement, and one (.8%) to an apicoectomy. On the other hand, 30 cases (25.2%) occurred spontaneously without any apparent dental disease, treatment, or trauma.13
Both Novartis Pharmaceuticals Corporation and the Food and Drug Administration have issued drug precautions for health professionals regarding osteonecrosis of the jaws. In 2004, Novartis made corresponding changes to the Precautions sections of its Zometa† and Aredia† labels.
The initial oral lesion seen in a case of bisphosphonate-associated necrosis of the jaws is a mucosal dehiscence, with exposure of the underlying mandible or maxilla (Fig. 2).
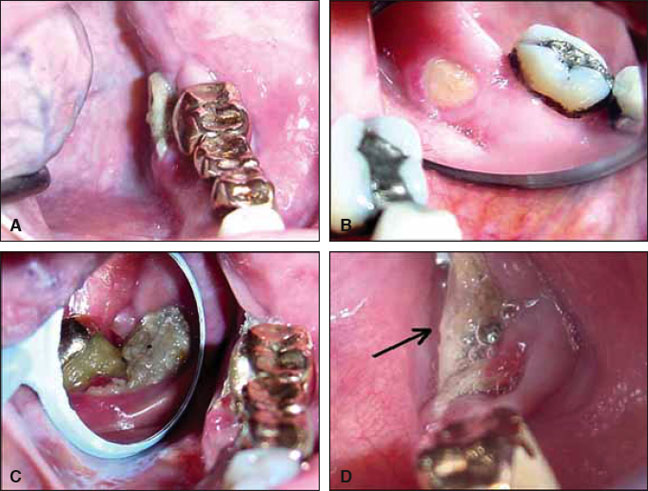
Fig. 2 Spontaneous osteonecrosis at mylohyoid plate, present for months in patient with metastatic breast carcinoma who was taking zoledronic acid. A. Initial presentation on left mandible. B. Initial presentation on right mandible. C. Progress of bone exposure one year later, showing bone necrosis and secondary infection on left mandible after extraction of upper right third molar and part of fixed bridge. D. Left mandible one month before patient’s death. (Reprinted with permission.2)
Although the lesion itself is reportedly quite painful, some patients have first noted irritation of adjacent structures, such as the lateral border of the tongue, due to constant abrasion from the exposed bone. What is most disturbing about this type of lesion is that it does not respond well to any known treatment regimen. Surgical debridement results in more necrotic bone and further deterioration. Cessation of the bisphosphonate therapy will not improve the situation, probably due to the persistence of the compound in bone. Even treatment with hyperbaric oxygen, which is beneficial in treating osteoradionecrosis, is of no benefit with bisphosphonate-induced bone exposure.13
Orthodontic Implications
Millions of peri- and postmenopausal women are currently taking oral bisphosphonates at the recommendation of their physicians for the prevention of skeletal events related to osteoporosis. Tens of thousands of patients are also receiving bisphosphonate therapy as part of their chemotherapeutic regimens for the treatment of malignant diseases. As we continue to treat an aging population, it is incumbent upon orthodontists to be acutely aware of the potential impact of this class of drugs on our patients.
In my own practice, I have changed my health history form to identify patients who are taking bisphosphonates, whether for cancer therapy or prevention of osteoporosis. I certainly do not include invasive laser therapy or miniscrew anchorage in the treatment plans for those who are currently receiving intravenous administration of bisphosphonates, nor will I recommend extractions.
In fact, orthodontic treatment itself must come into question with these patients. Kim and colleagues14 and Igarashi and colleagues15 have found that experimental animals challenged with bisphosphonates, either in systemic or topical form, demonstrated enhanced resistance to orthodontic relapse. This evidence suggests that tooth movement in patients receiving parenteral bisphosphonate therapy may be retarded. If prolonged orthodontic treatment is undertaken, are we increasing the potential for osteonecrosis of the jaws? Additionally, does our treatment plan change if a patient is diagnosed with cancer during treatment, as has happened in my practice? Do we discontinue treatment with less-than-ideal results, or do we carry on with heightened vigilance?
The ADA Council on Scientific Affairs recently published on its website an expert panel's recommendations for dental management of patients on oral bisphosphonate therapy16 (see the Editor's Corner, p. 403). Every orthodontist should consider this report required reading.
Conclusion
The increasing popularity of bisphosphonate drugs requires all of us to be cautious. The evidence is inconclusive at this point as to how long a patient must take oral bisphosphonates before the risk of osteonecrosis becomes high. It becomes a matter of clinical judgment on our part as to the treatment and level of invasiveness that we are willing to tolerate with this group of patients.
FOOTNOTES
- **Registered trademark of Procter & Gamble Company, Box 599, Cincinnati, OH 45201.
- ***Registered trademark of Sanofi-aventis, 300 Somerset Corporate Building, Bridgewater, NJ 08807.
- †Registered trademark of Novartis Pharmaceuticals Corp., 1 Health Plaza, East Hanover, NJ 07936.
- ‡Registered trademark of Merck & Co., Inc., 1 Merck Drive, Whitehouse Station, NJ 08889.
- ††Registered trademark of Roche Pharmaceuticals, 340 Kingland St., Nutley, NJ 07110.
REFERENCES
- 1. Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; and Engroff, S.L.: Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases, J. Oral Maxillofac. Surg. 62:527-534, 2004.
- 2. Migliorati, C.A.; Schubert, M.M.; Peterson, D.E.; and Seneda, L.M.: Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: An emerging oral complication of supportive cancer therapy, Cancer 104:83-93, 2005.
- 3. Rogers, M.J.; Watts, D.J.; and Russell, R.G.: Overview of bisphosphonates, Cancer 80(suppl. 8):1652-1660, 1997.
- 4. Licata, A.A.: Discovery, clinical development, and therapeutic uses of bisphosphonates, Ann. Pharmacother. 39:668-677, 2005.
- 5. Watts, N.B.: Treatment of osteoporosis with bisphosphonates, Endocrinol. Metab. Clin. N. Am. 27:419-439, 1998.
- 6. Russell, R.G.; Rogers, M.J.; Frith, J.C.; Luckman, S.P.; Coxon, F.P.; Benford, H.L.; Croucher, P.I.; Shipman, C.; and Fleisch, H.A.: The pharmacology of bisphosphonates and new insights into their mechanisms of action, J. Bone Miner. Res. 14(suppl. 2):53-65, 1999.
- 7. Fournier, P.; Boissier, S.; Filleur, S.; Guglielmi, J.; Cabon, F.; Colombel, M.; and Clezardin, P.: Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats, Cancer Res. 62:6538-6544, 2002.
- 8. Hortobagyi, G.N.; Theriault, R.L.; Lipton, A.; Porter, L.; Blayney, D.; Sinoff, C.; Wheeler, H.; Simeone, J.F.; Seaman, J.J.; Knight, R.D.; Heffernan, M.; Mellars, K.; and Reitsma, D.J.: Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate, J. Clin. Oncol. 16:2038-2044, 1998.
- 9. Conte, P.F.; Giannessi, P.G.; Latreille, J.; Mauriac, L.; Koliren, L.; Calabresi, F.; and Ford, J.M.: Delayed progression of bone metastases with pamidronate therapy in breast cancer patients: A randomized, multicenter phase III trial, Ann. Oncol. 5(suppl. 7):S41-44, 1994.
- 10. Berenson, J.R.; Lichtenstein, A.; Porter, L.; Dimopoulos, M.A.; Bordoni, R.; George, S.; Lipton, A.; Keller, A.; Ballester, O.; Kovacs, M.; Blacklock, H.; Bell, R.; Simeone, J.F.; Reitsma, D.J.; Heffernan, M.; Seaman, J.; and Knight, R.D.: Long term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events, J. Clin. Oncol. 16:593-602, 1998.
- 11. Theriault, R.L.; Lipton, A.; Hortobagyi, G.N.; Leff, R.; Gluck, S.; Stewart, J.F.; Costello, S.; Kennedy, I.; Simeone, J.; Seaman, J.J.; Knight, R.D.; Mellars, K.; Heffernan, M.; and Reitsma, D.J.: Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial, J. Clin. Oncol. 17:846-854, 1999.
- 12. Brumsen, C.; Hamdy, N.A.; and Papapoulos, S.E.: Long-term effects of bisphosphonates on the growing skeleton: Studies of young patients with severe osteoporosis, Med. 76:266-283, 1997.
- 13. Marx, R.E.; Sawatari, Y.; Fortin, M.; and Broumand, V.: Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment, J. Oral Maxillofac. Surg. 63:1567-1575, 2005.
- 14. Kim, T.W.; Yoshida, Y.; Yokoya, K.; and Sasaki, T.: An ultrastructural study of the effects of bisphosphonate administration on osteoclastic bone resorption during relapse of experimentally moved rat molars, Am. J. Orthod. 115:645-653, 1999.
- 15. Igarashi, K.; Mitani, H.; Adachi, H.; and Shinoda, H.: Anchorage and retentive effects of a bisphosphonate (AHBuBP) on tooth movements in rats, Am. J. Orthod. 106:279-289, 1994.
- 16. ADA Council on Scientific Affairs: Expert panel recommendation: Dental management of patients on oral bisphosphonate therapy, June 2006, http://www.ada.org/prof/resources/topics/topics_osteonecrosis_recommendations.pdf.


