Ceramic Brackets
The recent introduction of ceramic brackets to orthodontics is only part of the rapidly expanding ceramic technology in many industries. The computer's brain is built from the silicon chip--a ceramic. Telephone conversations are transmitted optically on a thin glass rod--a ceramic. Laser light is generated by a crystal, and its beams are directed, focused, and refracted by highly purified ceramic crystals. Electronic watches are synchronized by quartz crystals. Automobile engine prototypes made of ceramics are capable of operating at more efficient, higher temperatures without lubrication. Superconductors--ceramics capable of transmitting electrical energy without energy loss--are on the verge of dramatically altering our world.
Ceramics are a broad class of materials that include precious stones, glasses, clays, mixtures of ceramic compounds, and metallic oxides. In essence, a ceramic is neither metallic nor polymeric.
Ceramics are renowned for their hardness and for their resistance to high temperatures and to chemical degradation. The atomic structure that imparts these advantages also accounts for the most glaring fault of ceramics--their brittleness.
Metals can be deformed considerably without fracturing, even in the presence of significant impurities and at sharp intersections. This ductility is a function of their non-oxidized atomic structure. When metals are stressed, a shifting at grain boundaries causes a redistribution and relief of the stress.
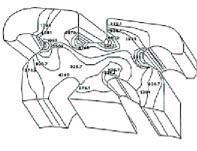
In contrast, ceramics used in orthodontic brackets have highly localized, directional atomic bonds (Fig. 1). This oxidized atomic lattice does not permit shifting of bonds and redistribution of stress. When stresses reach critical levels, the interatomic bonds break and material failure occurs. This is called "brittle failure".
Ceramic compounds, unlike metals, are also susceptible to crack propagation caused by minute imperfections or material impurities. High-strength ceramics can fail relatively easily when cracks or imperfections allow for local concentrations of stresses.
Although sharp intersections can be tolerated by stainless steel alloys, the geometry of ceramic brackets is critical in preventing stress build-up and brittle failure. Finite element analysis, an engineering application of computer models to analyze stress levels and distribution, has been used to design ceramic brackets (Fig. 2). Rounded intersections minimize the likelihood of brittle failure occurring at force levels substantially below the material's maximum strength.
Polycrystalline and Single-Crystal Ceramics
All the currently available ceramic brackets are composed of aluminum oxide. One type is polycrystalline, made of sintered or fused aluminum oxide particles, and the other contains a single crystal of aluminum oxide (Table 1).
Polycrystalline brackets are manufactured by blending aluminum oxide particles with a binder so that the mixture can be molded into a shape from which a bracket can be cut. The molded mixture is heated to temperatures in excess of 1,800°C to burn out the binder and fuse the particles together. This fused part is then machined with diamond cutting tools to provide the slot dimensions and other critical tolerances. The machined bracket must be heat-treated to remove surface imperfections and relieve stresses created by the cutting operations.
Optical properties and strength are incompatible for polycrystalline ceramics. The larger the ceramic grains, the greater the clarity or translucency. However, the material tends to become weaker when the grains reach a size of about 30 microns. Polycrystalline brackets begin as aluminum oxide particles of about .3 microns, which are fused to produce ceramic grains of 20-30 microns. Heat treatments after machining must be carefully controlled to prevent further grain fusion, which could detract from physical properties.
The main advantage of the polycrystalline manufacturing process is its ability to mold brackets--a relatively inexpensive operation that yields large quantities. The disadvantages of this molding process are the presence of structural imperfections at grain boundaries or of trace amounts of impurities. Impurities in quantities as minute as .001% or slight imperfections can serve as foci for crack propagation under stress.
The molding process and heat treatment produce fused aluminum oxide with grain boundaries that refract light, resulting in a degree of opacity. The most apparent difference between polycrystalline and single-crystal brackets is in their optical clarity.
Single-crystal ceramic brackets are manufactured by an entirely different process. Single crystals of man-made sapphire are produced by making a molten mass of aluminum oxide at temperatures in excess of 2,100°C. This mass is slowly cooled to allow a carefully controlled crystallization. The resultant crystal is purer than its natural counterpart.
Orthodontic manufacturers purchase these large single crystals and mill them into the shapes and dimensions of various brackets, using ultrasonic cutting techniques, diamond cutting, or a combination of the two. After milling, the sapphire crystals are heat-treated to remove surface imperfections and to relieve stresses induced by the milling operations.
The primary advantage of single-crystal manufacturing is the elimination of possible stress-inducing impurities or imperfections. The disadvantage is the difficulty and added expense of milling the third-hardest known material. This involves the development of new technology--with its inherent new problems. Production of polycrystalline brackets is less difficult, and hence these brackets are more readily available at present.
Single-crystal brackets have noticeably more optical clarity than polycrystalline brackets (Fig. 3). Whether the difference is significant is a judgment to be made by each clinician and patient.
Physical Properties
Little information has been generated on the physical properties of the finished products, but comparison of the generic materials is possible (Table 2).1-3 These data have several limitations:
1. They do not account for the geometry of the various brackets, cross-sections, and designs.
2. They do not include variations in manufacturing processes.
3. They do not consider the influences of stress rate on brittle failure.
4. They may not be clinically significant in terms of orthodontic use.
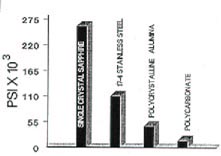
Ceramic brackets are touted as being harder than stainless steel (Fig. 4). Indeed, stainless steel is machined with aluminum oxide cutting wheels (Carborundum). Only diamond, pure carbon crystals, and boron carbide (a synthetic) are hard enough to machine aluminum oxide. However, a bracket harder than the time-tested stainless steel does not seem to have any clinical advantage. The hardness could even be detrimental if one has to grind away ceramic with a diamond instrument when a bracket fractures during debonding.
There is apparently a significant difference in tensile strength between ceramics and stainless steel (Fig. 5). This ability to resist structural failure is important, but the data generated thus far still have the limitations listed above. Laboratory testing of finished ceramic brackets, coupled with considerably more clinical experience than is now available, will be necessary to generate meaningful comparisons.
Both single-crystal and polycrystalline brackets resist staining or discoloration from any chemical substance likely to be encountered in the mouth. Unfortunately, the same cannot be said for elastic ligatures. And, because aluminum oxide is chemically inert, it cannot be directly adhered to by the acrylic materials used for orthodontic bonding.
Bonding Ceramic Brackets
Two different mechanisms are presently being used for bonding ceramic brackets: a mechanical retention via indentations and/or undercuts in the bracket base, and a chemical bonding using an adhesive intermediate.
Laboratory testing of mechanical retention indicates that adhesive-to-bracket bond strengths are less than those of equivalent-size foil/mesh metal brackets. Ceramic bracket bases have considerably fewer mechanical undercuts than are found in mesh base designs, and therefore the ceramic brackets might be expected to have greater bond failure rates. Although this lack of bond strength could be detrimental on lower teeth, it does not appear to be so on upper incisors, where ceramics are most beneficial esthetically. Debonding is much easier with a mechanical interlock because bond strengths are apparently marginal.
Chemical bonding, a more recent development, is used for "A"-Company's Starfire and Unitek's Transcend ceramic brackets. Glass is added to the aluminum oxide base and treated with a silane coupling agent. The silane bonds with the glass and has a free end of its molecules that will react with any of the acrylic bonding materials. The same chemical bonding mechanism is used for porcelain crowns and restorations. It produces exceptional bond strengths, but these can possibly exceed the brittle fracture resistance of the thinner areas of a ceramic bracket. The stresses of debonding can also be shifted from the bracket-adhesive interface to the adhesive-enamel interface.
When stainless steel brackets are bonded with mechanical retention, the ability of the relatively flexible metal base to absorb impact loads and to distort upon debonding causes a fracture of the bonding resin at the bracket base. A rigid, brittle ceramic bracket bonded to rigid, brittle enamel has little ability to absorb stresses. If the bracket-to-adhesive bond is too strong, then failure can only occur within the ceramic, within the adhesive, or within the enamel. A sudden impact loading is more likely to cause failure in the more brittle ceramic and enamel than in the polymeric bonding material.
Metal bonded brackets have succeeded because of a designed failure threshold. Using a bracket-to-adhesive interface whose bond strength exceeds those of current foil/mesh designs enters an untested area. Laboratory testing might not predict clinical failure when variables of enamel integrity, force levels, and adhesive thickness are involved.
Bonding Materials
Using a no-mix or one-step bonding material with ceramic brackets is probably contra-indicated. The no-mix materials contain higher concentrations of amine polymerization accelerator to achieve rapid setting. Therefore, the materials tend to discolor more than those of a two-component mixture. The no-mix systems were designed for thin glue lines and mesh bases, and the larger mechanical grooves and indentations in the mechanically retained ceramic bases may exceed their capability. Also, the polymerization shrinkage of no-mix systems could be too great for these relatively large undercuts and indentations.
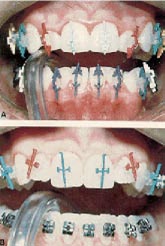
The newer light-cured restorative materials may prove to be the adhesives of choice. They offer excellent color stability and as much time as needed to position the brackets before polymerization. Brackets are preloaded with the light-cured paste while the teeth are prepared. After etching, the teeth can be coated with a light-cured sealant if desired. Each bracket is then applied to the tooth and pressed firmly in its approximate location. Excess adhesive is removed, and the bracket is adjusted, then tacked in place with a five-second exposure to the curing light. Once all the brackets have been positioned and tacked in place, they are cured with a 30-second exposure.
Extra caution should be exercised when direct bonding these small, radiolucent brackets. They are difficult to grasp with a placement instrument and can shoot out--perhaps resulting in a totally radiolucent bracket being deflected down the throat and aspirated.
Debonding
Debonding ceramic brackets that use a mechanical interlock does not appear to offer any new difficulties. The archwire is left in place, and each bracket is removed with a pin and ligature cutter or a pincer-type bond removal plier. The principle of applying a slow, peeling force at the bracket base still applies. Compressing the wings together--the least traumatic method for metal twin brackets--will only result in a brittle fracture of the ceramic bracket.
I recommend the same approach for debonding a ceramic bracket with a chemically treated base. Unitek suggests using a new torsional debonding instrument.4 My opinion, however, is that attempting to rotate a bracket off the tooth distributes the force over the entire base area, thus increasing the load to the adhesive-enamel interface and the risk of enamel damage. A slow, gradual compression mesiodistal to the base would seem to offer the best chance for inducing crack propagation within the bonding adhesive rather than the enamel.
Placement Aids and Ligatures
Plastic placement caps are offered with several of the ceramic brackets to assist in identification and alignment (Fig. 6). Some practice is required--preferably on a model or typodont--to become familiar with each manufacturer's design. Depending on the clinician's bonding expertise, the placement caps may not be too helpful in height and angulation positioning.
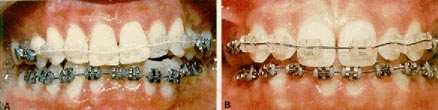
Steel or elastic hook ligatures can be used with care (Fig. 7). However, stress induced by steel ligatures can cause brittle failure.
Clear elastic (polyurethane) ligatures are indicated to maximize the esthetic appeal of ceramic brackets. The newer ceramic brackets have optical properties that tend to reflect and even magnify the color of elastic ligatures. Unfortunately, the currently available ligatures do not resist staining. Mustard, for example, can turn a clear ligature to a bright, almost fluorescent yellow. Patients should be forewarned about this discoloration, which will of course require more frequent replacements. Manufacturers are working on a solution to the ligature problem.
Conclusion
New designs of ceramic brackets are now being produced. They offer excellent optical properties and the promise of additional esthetic appeal without significant functional compromise. They are still not as strong or as durable as stainless steel, and ceramics will always be brittle by nature. Only optimum design, purity, and absence of manufacturing flaws can overcome the inherent brittleness and maximize ceramics' physical properties.
Concerns about bonding and debonding will undoubtedly be resolved with time. As occurred with metal brackets, design differences will probably diminish in favor of an optimal configuration.